Signaling by p38 MAPK stimulates nuclear localization of the microprocessor component p68 for processing of selected primary microRNAs
- PMID: 23482664
- PMCID: PMC3820758
- DOI: 10.1126/scisignal.2003706
Signaling by p38 MAPK stimulates nuclear localization of the microprocessor component p68 for processing of selected primary microRNAs
Abstract
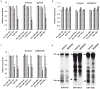
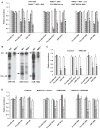
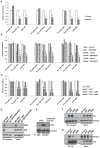
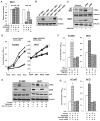
The importance of microRNAs (miRNAs) in biological and disease processes necessitates a better understanding of the mechanisms that regulate miRNA abundance. We showed that the activities of the mitogen-activated protein kinase (MAPK) p38 and its downstream effector kinase MAPK-activated protein kinase 2 (MK2) were necessary for the efficient processing of a subset of primary miRNAs (pri-miRNAs). Through yeast two-hybrid screening, we identified p68 (also known as DDX5), a key component of the Drosha complex that processes pri-miRNAs, as an MK2-interacting protein, and we found that MK2 phosphorylated p68 at Ser(197) in cells. In wild-type mouse embryonic fibroblasts (MEFs) treated with a p38 inhibitor or in MK2-deficient (MK2(-/-)) MEFs, expression of a phosphomimetic mutant p68 fully restored pri-miRNA processing, suggesting that MK2-mediated phosphorylation of p68 was essential for this process. We found that, whereas p68 was present in the nuclei of wild-type MEFs, it was found mostly in the cytoplasm of MK2(-/-) MEFs. Nuclear localization of p68 depended on MK2-mediated phosphorylation of Ser(197). In addition, inhibition of p38 MAPK promoted the growth of wild-type MEFs and breast cancer MCF7 cells by enhancing the abundance of c-Myc through suppression of the biogenesis of the miRNA miR-145, which targets c-Myc. Because pri-miRNA processing occurs in the nucleus, our findings suggest that the p38 MAPK-MK2 signaling pathway promotes miRNA biogenesis by facilitating the nuclear localization of p68.
Conflict of interest statement
Figures


Similar articles
-
Distinct cellular functions of MK2.Mol Cell Biol. 2002 Jul;22(13):4827-35. doi: 10.1128/MCB.22.13.4827-4835.2002. Mol Cell Biol. 2002. PMID: 12052889 Free PMC article.
-
Posttranslational regulation of tristetraprolin subcellular localization and protein stability by p38 mitogen-activated protein kinase and extracellular signal-regulated kinase pathways.Mol Cell Biol. 2006 Mar;26(6):2408-18. doi: 10.1128/MCB.26.6.2408-2418.2006. Mol Cell Biol. 2006. PMID: 16508015 Free PMC article.
-
IL-1β-induced and p38MAPK-dependent activation of the mitogen-activated protein kinase-activated protein kinase 2 (MK2) in hepatocytes: Signal transduction with robust and concentration-independent signal amplification.J Biol Chem. 2017 Apr 14;292(15):6291-6302. doi: 10.1074/jbc.M117.775023. Epub 2017 Feb 21. J Biol Chem. 2017. PMID: 28223354 Free PMC article.
-
Mitogen-activated protein kinase p38 and MK2, MK3 and MK5: ménage à trois or ménage à quatre?Cell Signal. 2010 Aug;22(8):1185-92. doi: 10.1016/j.cellsig.2010.03.002. Epub 2010 Mar 11. Cell Signal. 2010. PMID: 20227494 Review.
-
Mitogen-activated protein kinase-activated protein kinase 2 in neuroinflammation, heat shock protein 27 phosphorylation, and cell cycle: role and targeting.Mol Pharmacol. 2014 Feb;85(2):345-56. doi: 10.1124/mol.113.090365. Epub 2013 Dec 2. Mol Pharmacol. 2014. PMID: 24296859 Review.
Cited by
-
Suppression of lung cancer progression by isoliquiritigenin through its metabolite 2, 4, 2', 4'-Tetrahydroxychalcone.J Exp Clin Cancer Res. 2018 Oct 3;37(1):243. doi: 10.1186/s13046-018-0902-4. J Exp Clin Cancer Res. 2018. PMID: 30285892 Free PMC article.
-
MicroRNA-125b-5p regulates IL-1β induced inflammatory genes via targeting TRAF6-mediated MAPKs and NF-κB signaling in human osteoarthritic chondrocytes.Sci Rep. 2019 May 3;9(1):6882. doi: 10.1038/s41598-019-42601-3. Sci Rep. 2019. PMID: 31053727 Free PMC article.
-
Pan-cancer analysis of the prognostic and immunological roles of DEAD-box helicase 5 (DDX5) in human tumors.Front Genet. 2022 Oct 13;13:1039440. doi: 10.3389/fgene.2022.1039440. eCollection 2022. Front Genet. 2022. PMID: 36313454 Free PMC article.
-
LRRK2 Contributes to Secondary Brain Injury Through a p38/Drosha Signaling Pathway After Traumatic Brain Injury in Rats.Front Cell Neurosci. 2018 Mar 1;12:51. doi: 10.3389/fncel.2018.00051. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29545743 Free PMC article.
-
miRNA biogenesis: biological impact in the development of cancer.Cancer Biol Ther. 2014;15(11):1444-55. doi: 10.4161/15384047.2014.955442. Cancer Biol Ther. 2014. PMID: 25482951 Free PMC article. Review.
References
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
-
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. - PubMed
-
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16:223–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

