Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire
- PMID: 23482645
- PMCID: PMC3654747
- DOI: 10.1093/infdis/jit098
Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire
Abstract
Background: The licensing of herpes zoster vaccine has demonstrated that therapeutic vaccination can help control chronic viral infection. Unfortunately, human trials of immunodeficiency virus (HIV) vaccine have shown only marginal efficacy.
Methods: In this double-blind study, 17 HIV-infected individuals with viral loads of <50 copies/mL and CD4(+) T-cell counts of >350 cells/µL were randomly assigned to the vaccine or placebo arm. Vaccine recipients received 3 intramuscular injections of HIV DNA (4 mg) coding for clade B Gag, Pol, and Nef and clade A, B, and C Env, followed by a replication-deficient adenovirus type 5 boost (10(10) particle units) encoding all DNA vaccine antigens except Nef. Humoral, total T-cell, and CD8(+) cytotoxic T-lymphocyte (CTL) responses were studied before and after vaccination. Single-copy viral loads and frequencies of latently infected CD4(+) T cells were determined.
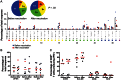
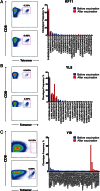
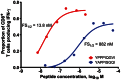
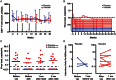
Results: Vaccination was safe and well tolerated. Significantly stronger HIV-specific T-cell responses against Gag, Pol, and Env, with increased polyfunctionality and a broadened epitope-specific CTL repertoire, were observed after vaccination. No changes in single-copy viral load or the frequency of latent infection were observed.
Conclusions: Vaccination of individuals with existing HIV-specific immunity improved the magnitude, breadth, and polyfunctionality of HIV-specific memory T-cell responses but did not impact markers of viral control.
Clinical trials registration: NCT00270465.
Keywords: HIV; cytotoxic T lymphocytes; humoral immunity; therapy; vaccination; viral latency.
Figures

Similar articles
-
A Randomized Placebo-Controlled Efficacy Study of a Prime Boost Therapeutic Vaccination Strategy in HIV-1-Infected Individuals: VRI02 ANRS 149 LIGHT Phase II Trial.J Virol. 2021 Apr 12;95(9):e02165-20. doi: 10.1128/JVI.02165-20. Print 2021 Apr 12. J Virol. 2021. PMID: 33568510 Free PMC article. Clinical Trial.
-
Antigenic competition in CD4+ T cell responses in a randomized, multicenter, double-blind clinical HIV vaccine trial.Sci Transl Med. 2019 Nov 20;11(519):eaaw1673. doi: 10.1126/scitranslmed.aaw1673. Sci Transl Med. 2019. PMID: 31748227 Free PMC article. Clinical Trial.
-
A Phase I Randomized Therapeutic MVA-B Vaccination Improves the Magnitude and Quality of the T Cell Immune Responses in HIV-1-Infected Subjects on HAART.PLoS One. 2015 Nov 6;10(11):e0141456. doi: 10.1371/journal.pone.0141456. eCollection 2015. PLoS One. 2015. PMID: 26544853 Free PMC article. Clinical Trial.
-
Therapeutic immunization for HIV.Springer Semin Immunopathol. 2006 Nov;28(3):221-30. doi: 10.1007/s00281-006-0029-0. Epub 2006 Oct 10. Springer Semin Immunopathol. 2006. PMID: 17031650 Review.
-
Unusual antigen presentation offers new insight into HIV vaccine design.Curr Opin Immunol. 2017 Jun;46:75-81. doi: 10.1016/j.coi.2017.04.009. Epub 2017 May 12. Curr Opin Immunol. 2017. PMID: 28505602 Free PMC article. Review.
Cited by
-
How Might We Cure HIV?Curr Infect Dis Rep. 2014 Mar;16(3):392. doi: 10.1007/s11908-014-0392-2. Curr Infect Dis Rep. 2014. PMID: 24562540
-
Adenovirus vector-induced CD8⁺ T effector memory cell differentiation and recirculation, but not proliferation, are important for protective immunity against experimental Trypanosoma cruzi Infection.Hum Gene Ther. 2014 Apr;25(4):350-63. doi: 10.1089/hum.2013.218. Epub 2014 Mar 31. Hum Gene Ther. 2014. PMID: 24568548 Free PMC article.
-
Evaluating the efficacy of therapeutic HIV vaccines through analytical treatment interruptions.J Int AIDS Soc. 2015 Nov 9;18(1):20497. doi: 10.7448/IAS.18.1.20497. eCollection 2015. J Int AIDS Soc. 2015. PMID: 26561337 Free PMC article. Review.
-
Emerging strategies to deplete the HIV reservoir.Curr Opin Infect Dis. 2014 Feb;27(1):29-35. doi: 10.1097/QCO.0000000000000026. Curr Opin Infect Dis. 2014. PMID: 24296585 Free PMC article. Review.
-
Effect of therapeutic intensification followed by HIV DNA prime and rAd5 boost vaccination on HIV-specific immunity and HIV reservoir (EraMune 02): a multicentre randomised clinical trial.Lancet HIV. 2015 Mar;2(3):e82-91. doi: 10.1016/S2352-3018(15)00026-0. Epub 2015 Feb 17. Lancet HIV. 2015. PMID: 26424549 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

