Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension
- PMID: 23479634
- PMCID: PMC3637763
- DOI: 10.1073/pnas.1220697110
Optically triggering spatiotemporally confined GPCR activity in a cell and programming neurite initiation and extension
Abstract
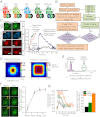
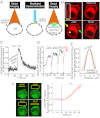
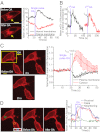
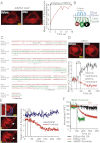
G-protein-coupled receptor (GPCR) activity gradients evoke important cell behavior but there is a dearth of methods to induce such asymmetric signaling in a cell. Here we achieved reversible, rapidly switchable patterns of spatiotemporally restricted GPCR activity in a single cell. We recruited properties of nonrhodopsin opsins--rapid deactivation, distinct spectral tuning, and resistance to bleaching--to activate native Gi, Gq, or Gs signaling in selected regions of a cell. Optical inputs were designed to spatiotemporally control levels of second messengers, IP3, phosphatidylinositol (3,4,5)-triphosphate, and cAMP in a cell. Spectrally selective imaging was accomplished to simultaneously monitor optically evoked molecular and cellular response dynamics. We show that localized optical activation of an opsin-based trigger can induce neurite initiation, phosphatidylinositol (3,4,5)-triphosphate increase, and actin remodeling. Serial optical inputs to neurite tips can refashion early neuron differentiation. Methods here can be widely applied to program GPCR-mediated cell behaviors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Live Cell Imaging and Optogenetics-Based Assays for GPCR Activity.Methods Mol Biol. 2021;2268:207-221. doi: 10.1007/978-1-0716-1221-7_14. Methods Mol Biol. 2021. PMID: 34085271
-
Detection of G protein-selective G protein-coupled receptor (GPCR) conformations in live cells.J Biol Chem. 2013 Jun 14;288(24):17167-78. doi: 10.1074/jbc.M113.464065. Epub 2013 Apr 29. J Biol Chem. 2013. PMID: 23629648 Free PMC article.
-
Optical control demonstrates switch-like PIP3 dynamics underlying the initiation of immune cell migration.Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):E1575-83. doi: 10.1073/pnas.1220755110. Epub 2013 Apr 8. Proc Natl Acad Sci U S A. 2013. PMID: 23569254 Free PMC article.
-
Real-time monitoring of GPCR/cAMP signalling by FRET and single-molecule microscopy.Horm Metab Res. 2014 Nov;46(12):827-32. doi: 10.1055/s-0034-1384523. Epub 2014 Jul 23. Horm Metab Res. 2014. PMID: 25054437 Review.
-
Optical techniques to analyze real-time activation and signaling of G-protein-coupled receptors.Trends Pharmacol Sci. 2008 Mar;29(3):159-65. doi: 10.1016/j.tips.2007.12.002. Epub 2008 Feb 11. Trends Pharmacol Sci. 2008. PMID: 18262662 Review.
Cited by
-
Targeted Proteomics-Driven Computational Modeling of Macrophage S1P Chemosensing.Mol Cell Proteomics. 2015 Oct;14(10):2661-81. doi: 10.1074/mcp.M115.048918. Epub 2015 Jul 21. Mol Cell Proteomics. 2015. PMID: 26199343 Free PMC article.
-
A photoswitchable GPCR-based opsin for presynaptic inhibition.Neuron. 2021 Jun 2;109(11):1791-1809.e11. doi: 10.1016/j.neuron.2021.04.026. Epub 2021 May 11. Neuron. 2021. PMID: 33979635 Free PMC article.
-
In-silico predicted mouse melanopsins with blue spectral shifts deliver efficient subcellular signaling.Cell Commun Signal. 2024 Aug 8;22(1):394. doi: 10.1186/s12964-024-01753-0. Cell Commun Signal. 2024. PMID: 39118111 Free PMC article.
-
Single-cell analysis of G-protein signal transduction.J Biol Chem. 2015 Mar 13;290(11):6681-8. doi: 10.1074/jbc.R114.616391. Epub 2015 Jan 20. J Biol Chem. 2015. PMID: 25605723 Free PMC article. Review.
-
Innovative Optogenetic Strategies for Vision Restoration.Front Cell Neurosci. 2018 Sep 21;12:316. doi: 10.3389/fncel.2018.00316. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30297985 Free PMC article.
References
-
- Mogilner A, Allard J, Wollman R. Cell polarity: Quantitative modeling as a tool in cell biology. Science. 2012;336(6078):175–179. - PubMed
-
- Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9(2):187–195. - PubMed
-
- Frentiu FD, Briscoe AD. A butterfly eye’s view of birds. Bioessays. 2008;30(11–12):1151–1162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

