Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus
- PMID: 23477737
- PMCID: PMC4115271
- DOI: 10.1016/j.immuni.2013.02.012
Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus
Abstract
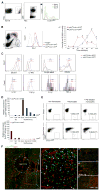
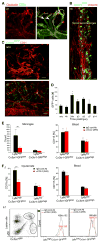
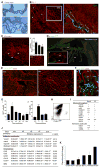
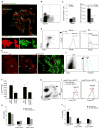
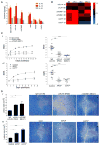
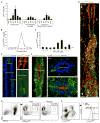
Monocyte-derived macrophages are essential for recovery after spinal cord injury, but their homing mechanism is poorly understood. Here, we show that although of common origin, the homing of proinflammatory (M1) and the "alternatively activated" anti-inflammatory (M2) macrophages to traumatized spinal cord (SC) was distinctly regulated, neither being through breached blood-brain barrier. The M1 macrophages (Ly6c(hi)CX3CR1(lo)) derived from monocytes homed in a CCL2 chemokine-dependent manner through the adjacent SC leptomeninges. The resolving M2 macrophages (Ly6c(lo)CX3CR1(hi)) derived from monocytes trafficked through a remote blood-cerebrospinal-fluid (CSF) barrier, the brain-ventricular choroid plexus (CP), via VCAM-1-VLA-4 adhesion molecules and epithelial CD73 enzyme for extravasation and epithelial transmigration. Blockage of these determinants, or mechanical CSF flow obstruction, inhibited M2 macrophage recruitment and impaired motor-function recovery. The CP, along with the CSF and the central canal, provided an anti-inflammatory supporting milieu, potentially priming the trafficking monocytes. Overall, our finding demonstrates that the route of monocyte entry to central nervous system provides an instructional environment to shape their function.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages.J Neurosci. 2011 Jul 6;31(27):9910-22. doi: 10.1523/JNEUROSCI.2114-11.2011. J Neurosci. 2011. PMID: 21734283 Free PMC article.
-
Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke.J Neuroinflammation. 2017 Jul 28;14(1):153. doi: 10.1186/s12974-017-0909-3. J Neuroinflammation. 2017. PMID: 28754163 Free PMC article.
-
Mobilisation of the splenic monocyte reservoir and peripheral CX₃CR1 deficiency adversely affects recovery from spinal cord injury.Exp Neurol. 2013 Sep;247:226-40. doi: 10.1016/j.expneurol.2013.05.002. Epub 2013 May 9. Exp Neurol. 2013. PMID: 23664962
-
Is neuroinflammation in the injured spinal cord different than in the brain? Examining intrinsic differences between the brain and spinal cord.Exp Neurol. 2014 Aug;258:112-20. doi: 10.1016/j.expneurol.2014.04.007. Exp Neurol. 2014. PMID: 25017892 Review.
-
Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders.Drug Discov Today. 2015 Aug;20(8):928-41. doi: 10.1016/j.drudis.2015.05.003. Epub 2015 May 13. Drug Discov Today. 2015. PMID: 25979470 Review.
Cited by
-
Dealing with Danger in the CNS: The Response of the Immune System to Injury.Neuron. 2015 Jul 1;87(1):47-62. doi: 10.1016/j.neuron.2015.05.019. Neuron. 2015. PMID: 26139369 Free PMC article. Review.
-
The Immune System's Role in the Consequences of Mild Traumatic Brain Injury (Concussion).Front Immunol. 2021 Feb 15;12:620698. doi: 10.3389/fimmu.2021.620698. eCollection 2021. Front Immunol. 2021. PMID: 33679762 Free PMC article. Review.
-
A cord blood monocyte-derived cell therapy product accelerates brain remyelination.JCI Insight. 2016 Aug 18;1(13):e86667. doi: 10.1172/jci.insight.86667. JCI Insight. 2016. PMID: 27699230 Free PMC article.
-
Positively Charged Oligo[Poly(Ethylene Glycol) Fumarate] Scaffold Implantation Results in a Permissive Lesion Environment after Spinal Cord Injury in Rat.Tissue Eng Part A. 2015 Jul;21(13-14):2099-114. doi: 10.1089/ten.TEA.2015.0019. Tissue Eng Part A. 2015. PMID: 25891264 Free PMC article.
-
Cancer-Associated Fibroblasts: The Origin, Biological Characteristics and Role in Cancer-A Glance on Colorectal Cancer.Cancers (Basel). 2022 Sep 9;14(18):4394. doi: 10.3390/cancers14184394. Cancers (Basel). 2022. PMID: 36139552 Free PMC article. Review.
References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
-
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. - PMC - PubMed
-
- Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WE, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

