Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2
- PMID: 23474247
- PMCID: PMC3674112
- DOI: 10.1016/j.ophtha.2012.11.048
Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2
Abstract
Objective: The aim of this study was to show the clinical data of long-term (3-year) follow-up of 5 patients affected by Leber congenital amaurosis type 2 (LCA2) treated with a single unilateral injection of adeno-associated virus AAV2-hRPE65v2.
Design: Clinical trial.
Participants: Five LCA2 patients with RPE65 gene mutations.
Methods: After informed consent and confirmation of trial eligibility criteria, the eye with worse visual function was selected for subretinal delivery of adeno-associated virus (AAV2-hRPE65v2). Subjects were evaluated before and after surgery at designated follow-up visits (1, 2, 3, 14, 30, 60, 90, 180, 270, and 365 days, 1.5 years, and 3 years) by complete ophthalmic examination. Efficacy for each subject was monitored with best-corrected visual acuity, kinetic visual field, nystagmus testing, and pupillary light reflex.
Main outcome measures: Best-corrected visual acuity, kinetic visual field, nystagmus testing, and pupillary light reflex.
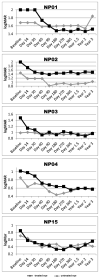
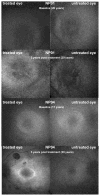
Results: The data showed a statistically significant improvement of best-corrected visual acuity between baseline and 3 years after treatment in the treated eye (P<0.001). In all patients, an enlargement of the area of visual field was observed that remained stable until 3 years after injection (average values: baseline, 1058 deg(2) vs. 3 years after treatment, 4630 deg(2)) and a reduction of the nystagmus frequency compared with baseline at the 3-year time point. Furthermore, a statistically significant difference was observed in the pupillary constriction of the treated eye (P<0.05) compared with the untreated eye in 3 patients at 1- and 3-year time points. No patients experienced serious adverse events related to the vector in the 3-year postinjection period.
Conclusions: The long-term follow-up data (3 years) on the 5-patient Italian cohort involved in the LCA2 gene therapy clinical trial clearly showed a stability of improvement in visual and retinal function that had been achieved a few months after treatment. Longitudinal data analysis showed that the maximum improvement was achieved within 6 months after treatment, and the visual improvement was stable up to the last observed time point.
Financial disclosure(s): Proprietary or commercial disclosure may be found after the references.
Copyright © 2013 American Academy of Ophthalmology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest: J.B. and A.M.M. are co-inventors on a patent (2797-11-US) for a method to treat or slow the development of blindness, but both waived any financial interest in this technology in 2002. K.A.H. and J.F.W. are co-inventors on a patent regarding methods of making AAV vectors for clinical studies (U.S. patent 61/299,184, 2010). J.B. and J.F.W. serve on the scientific advisory board for Avalanche Technologies. J.F.W. has consulted for Tacere Therapeutics, Genzyme, Novartis, and Genetix Inc. The other authors declare that they have no competing interests
Figures

Similar articles
-
Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years.Arch Ophthalmol. 2012 Jan;130(1):9-24. doi: 10.1001/archophthalmol.2011.298. Epub 2011 Sep 12. Arch Ophthalmol. 2012. PMID: 21911650 Free PMC article. Clinical Trial.
-
Results at 2 Years after Gene Therapy for RPE65-Deficient Leber Congenital Amaurosis and Severe Early-Childhood-Onset Retinal Dystrophy.Ophthalmology. 2016 Jul;123(7):1606-20. doi: 10.1016/j.ophtha.2016.03.003. Epub 2016 Apr 19. Ophthalmology. 2016. PMID: 27102010 Clinical Trial.
-
Results at 5 Years After Gene Therapy for RPE65-Deficient Retinal Dystrophy.Hum Gene Ther. 2018 Dec;29(12):1428-1437. doi: 10.1089/hum.2018.014. Epub 2018 Jul 24. Hum Gene Ther. 2018. PMID: 29869534 Clinical Trial.
-
Gene therapy for Leber congenital amaurosis: advances and future directions.Graefes Arch Clin Exp Ophthalmol. 2012 Aug;250(8):1117-28. doi: 10.1007/s00417-012-2028-2. Epub 2012 May 29. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 22644094 Free PMC article. Review.
-
Gene therapy for RPE65-related retinal disease.Ophthalmic Genet. 2018 Dec;39(6):671-677. doi: 10.1080/13816810.2018.1533027. Epub 2018 Oct 18. Ophthalmic Genet. 2018. PMID: 30335549 Review.
Cited by
-
RNA Interference Prevents Autosomal-Dominant Hearing Loss.Am J Hum Genet. 2016 Jun 2;98(6):1101-1113. doi: 10.1016/j.ajhg.2016.03.028. Epub 2016 May 26. Am J Hum Genet. 2016. PMID: 27236922 Free PMC article.
-
Efficient gene delivery to photoreceptors using AAV2/rh10 and rescue of the Rho(-/-) mouse.Mol Ther Methods Clin Dev. 2015 May 6;2:15016. doi: 10.1038/mtm.2015.16. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26029727 Free PMC article.
-
High-Level rAAV Vector Production by rAdV-Mediated Amplification of Small Amounts of Input Vector.Viruses. 2022 Dec 24;15(1):64. doi: 10.3390/v15010064. Viruses. 2022. PMID: 36680104 Free PMC article.
-
A Cross-Sectional and Longitudinal Study of Retinal Sensitivity in RPE65-Associated Leber Congenital Amaurosis.Invest Ophthalmol Vis Sci. 2018 Jul 2;59(8):3330-3339. doi: 10.1167/iovs.18-23873. Invest Ophthalmol Vis Sci. 2018. PMID: 30025081 Free PMC article.
-
Outcome Measures Used in Ocular Gene Therapy Trials: A Scoping Review of Current Practice.Front Pharmacol. 2019 Sep 18;10:1076. doi: 10.3389/fphar.2019.01076. eCollection 2019. Front Pharmacol. 2019. PMID: 31620003 Free PMC article. Review.
References
-
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. - PubMed
-
- Walia S, Fishman GA, Jacobson SG, et al. Visual acuity in patients with Leber’s congenital amaurosis and early childhood-onset retinitis pigmentosa. Ophthalmology. 2010;117:1190–8. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

