Phase I clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma
- PMID: 23470466
- PMCID: PMC3619266
- DOI: 10.1038/bjc.2013.90
Phase I clinical trial of human vascular endothelial growth factor receptor 1 peptide vaccines for patients with metastatic renal cell carcinoma
Abstract
Background: It is well known that renal cell carcinoma (RCC) represents one of the most immune-responsive cancers. Although the lack of defined antigens in RCC has hindered more specific vaccine development, research regarding vaccination therapy has been of special interest for the treatment of RCC for more than 30 years.
Methods: To evaluate the safety of the vascular endothelial growth factor receptor 1 (VEGFR1) peptide vaccination and its clinical outcomes, data from 18 metastatic RCC (mRCC) patients treated with VEGFR1 vaccine were collected. Toxicity assessments were performed. Clinical outcomes included assessment using CT scanning, magnetic resonance imaging or X-ray examination in accordance with the WHO Response Evaluation Criteria in Solid Tumors.
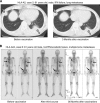
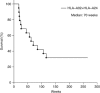
Results: No patient showed any toxicities of grade 3 or greater. Of the 18 patients, 2 patients showed a partial response during treatment. Stable disease for more than 5 months was observed in eight patients with a median duration of 16.5 months (4-32 months). At the time of the analysis in this study, six patients were alive with a median follow-up of 30 months (26-36 months).
Conclusion: These results suggest that VEGFR1 peptide vaccine is safe and is recommended for further trials for patients with mRCC.
Figures


Similar articles
-
Phase 1 trial of allogeneic gene-modified tumor cell vaccine RCC-26/CD80/IL-2 in patients with metastatic renal cell carcinoma.Hum Gene Ther. 2010 Mar;21(3):285-97. doi: 10.1089/hum.2008.192. Hum Gene Ther. 2010. PMID: 19788391 Clinical Trial.
-
A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma.Clin Cancer Res. 2006 Mar 15;12(6):1768-75. doi: 10.1158/1078-0432.CCR-05-2253. Clin Cancer Res. 2006. PMID: 16551861 Clinical Trial.
-
Expression level of vascular endothelial growth factor receptor-2 in radical nephrectomy specimens as a prognostic predictor in patients with metastatic renal cell carcinoma treated with sunitinib.Urol Oncol. 2013 May;31(4):493-8. doi: 10.1016/j.urolonc.2011.02.012. Epub 2011 Apr 7. Urol Oncol. 2013. PMID: 21478036
-
IMA901: a multi-peptide cancer vaccine for treatment of renal cell cancer.Hum Vaccin Immunother. 2014;10(11):3179-89. doi: 10.4161/21645515.2014.983857. Hum Vaccin Immunother. 2014. PMID: 25625928 Free PMC article. Review.
-
Vaccine therapy in patients with renal cell carcinoma.Eur Urol. 2009 Jun;55(6):1333-42. doi: 10.1016/j.eururo.2009.01.043. Epub 2009 Jan 30. Eur Urol. 2009. PMID: 19201522 Review.
Cited by
-
Current status of antigen-specific T-cell immunotherapy for advanced renal-cell carcinoma.Hum Vaccin Immunother. 2021 Jul 3;17(7):1882-1896. doi: 10.1080/21645515.2020.1870846. Epub 2021 Mar 5. Hum Vaccin Immunother. 2021. PMID: 33667140 Free PMC article. Review.
-
A novel kinase mutation in VEGFR-1 predisposes its αC-helix/activation loop towards allosteric activation: Atomic insights from protein simulation.Eur J Hum Genet. 2016 Aug;24(9):1287-93. doi: 10.1038/ejhg.2016.26. Epub 2016 Apr 6. Eur J Hum Genet. 2016. PMID: 27049304 Free PMC article.
-
A pilot study of peptide vaccines for VEGF receptor 1 and 2 in patients with recurrent/progressive high grade glioma.Oncotarget. 2018 Apr 20;9(30):21569-21579. doi: 10.18632/oncotarget.25131. eCollection 2018 Apr 20. Oncotarget. 2018. PMID: 29765561 Free PMC article.
-
Peptide-Based Treatment: A Promising Cancer Therapy.J Immunol Res. 2015;2015:761820. doi: 10.1155/2015/761820. Epub 2015 Oct 19. J Immunol Res. 2015. PMID: 26568964 Free PMC article. Review.
-
Clinical and histopathological analyses of VEGF receptors peptide vaccine in patients with primary glioblastoma - a case series.BMC Cancer. 2020 Mar 12;20(1):196. doi: 10.1186/s12885-020-6589-x. BMC Cancer. 2020. PMID: 32164575 Free PMC article. Clinical Trial.
References
-
- Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89:293–300. - PubMed
-
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. - PubMed
-
- Finn OJ, Lotze MT. A decade in the life of tumor immunology. Clin Cancer Res. 2001;7:759–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

