A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo
- PMID: 23468485
- PMCID: PMC3648190
- DOI: 10.1128/JVI.00281-13
A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo
Abstract
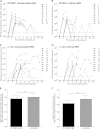
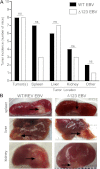
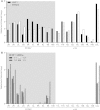
Over 90% of the adult human population is chronically infected with the Epstein-Barr virus (EBV), an oncogenic herpesvirus. EBV primarily infects naive human B cells and persists latently in memory B cells. Most individuals experience an asymptomatic infection that is effectively controlled by the adaptive immune response. However, EBV-associated lymphomas can develop in immunocompromised individuals. These tumors typically express all nine EBV latent proteins (latency III). Latency III is also associated with the expression of three precursor microRNAs (miRNAs) located within the EBV BHRF1 gene locus. The role of these BHRF1 miRNAs was unclear until recent in vitro studies demonstrated that they cooperate to enhance virus-induced B cell transformation and decrease the antigenic load of virus-infected cells, indicating that the BHRF1 miRNA cluster may serve as a novel therapeutic target for the treatment of latency III EBV-associated malignancies. However, to date, it is not known if BHRF1 miRNAs enhance virus-induced oncogenesis and/or immune evasion of EBV in vivo. To understand the in vivo contribution of the BHRF1 miRNA cluster to EBV infection and EBV-associated tumorigenesis, we monitored EBV infection and assessed tumor formation in humanized mice exposed to wild-type virus and a viral mutant (Δ123) that lacks all three BHRF1 miRNAs. Our results demonstrate that while the BHRF1 miRNAs facilitate the development of acute systemic EBV infection, they do not enhance the overall oncogenic potential of EBV in vivo.
Figures




Similar articles
-
A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus.PLoS Pathog. 2011 Feb;7(2):e1001294. doi: 10.1371/journal.ppat.1001294. Epub 2011 Feb 17. PLoS Pathog. 2011. PMID: 21379335 Free PMC article.
-
MicroRNAs of Epstein-Barr Virus Attenuate T-Cell-Mediated Immune Control In Vivo.mBio. 2019 Jan 15;10(1):e01941-18. doi: 10.1128/mBio.01941-18. mBio. 2019. PMID: 30647153 Free PMC article.
-
Knockout of Epstein-Barr virus BPLF1 retards B-cell transformation and lymphoma formation in humanized mice.mBio. 2015 Oct 20;6(5):e01574-15. doi: 10.1128/mBio.01574-15. mBio. 2015. PMID: 26489865 Free PMC article.
-
Epstein-Barr virus-encoded microRNAs as regulators in host immune responses.Int J Biol Sci. 2018 Apr 5;14(5):565-576. doi: 10.7150/ijbs.24562. eCollection 2018. Int J Biol Sci. 2018. PMID: 29805308 Free PMC article. Review.
-
Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases.Front Immunol. 2020 Mar 3;11:367. doi: 10.3389/fimmu.2020.00367. eCollection 2020. Front Immunol. 2020. PMID: 32194570 Free PMC article. Review.
Cited by
-
Gammaherpesvirus small noncoding RNAs are bifunctional elements that regulate infection and contribute to virulence in vivo.mBio. 2015 Feb 17;6(1):e01670-14. doi: 10.1128/mBio.01670-14. mBio. 2015. PMID: 25691585 Free PMC article.
-
Epstein-Barr virus latent genes.Exp Mol Med. 2015 Jan 23;47(1):e131. doi: 10.1038/emm.2014.84. Exp Mol Med. 2015. PMID: 25613728 Free PMC article. Review.
-
Pathobiologic Roles of Epstein-Barr Virus-Encoded MicroRNAs in Human Lymphomas.Int J Mol Sci. 2018 Apr 12;19(4):1168. doi: 10.3390/ijms19041168. Int J Mol Sci. 2018. PMID: 29649101 Free PMC article. Review.
-
Viral Encoded miRNAs in Tumorigenesis: Theranostic Opportunities in Precision Oncology.Microorganisms. 2022 Jul 18;10(7):1448. doi: 10.3390/microorganisms10071448. Microorganisms. 2022. PMID: 35889167 Free PMC article. Review.
-
Immunomodulatory roles of human herpesvirus-encoded microRNA in host-virus interaction.Rev Med Virol. 2020 Jan;30(1):e2081. doi: 10.1002/rmv.2081. Epub 2019 Aug 20. Rev Med Virol. 2020. PMID: 31432608 Free PMC article. Review.
References
-
- Rickinson AB, Kieff E. 2007. Epstein-Barr virus, p 2655–2700 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA
-
- Thorley-Lawson DA. 2005. EBV persistence and latent infection in vivo, p 309–357 In Robertson ES. (ed), Epstein-Barr virus. Caister Academic Press, Wymondham, Norfolk, England
-
- Heslop HE. 2005. Biology and treatment of Epstein-Barr virus-associated non-Hodgkin lymphomas. Hematology Am. Soc. Hematol. Educ. Program 2005:260–266 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

