Microglia regulate the number of neural precursor cells in the developing cerebral cortex
- PMID: 23467340
- PMCID: PMC3711552
- DOI: 10.1523/JNEUROSCI.3441-12.2013
Microglia regulate the number of neural precursor cells in the developing cerebral cortex
Abstract
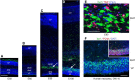
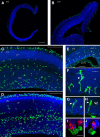
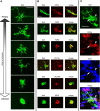
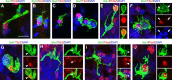
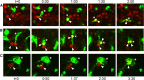
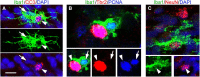
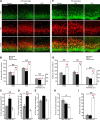
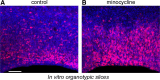
Neurogenesis must be properly regulated to ensure that cell production does not exceed the requirements of the growing cerebral cortex, yet our understanding of mechanisms that restrain neuron production remains incomplete. We investigated the function of microglial cells in the developing cerebral cortex of prenatal and postnatal macaques and rats and show that microglia limit the production of cortical neurons by phagocytosing neural precursor cells. We show that microglia selectively colonize the cortical proliferative zones and phagocytose neural precursor cells as neurogenesis nears completion. We found that deactivating microglia in utero with tetracyclines or eliminating microglia from the fetal cerebral cortex with liposomal clodronate significantly increased the number of neural precursor cells, while activating microglia in utero through maternal immune activation significantly decreased the number of neural precursor cells. These data demonstrate that microglia play a fundamental role in regulating the size of the precursor cell pool in the developing cerebral cortex, expanding our understanding of the mechanisms that regulate cortical development. Furthermore, our data suggest that any factor that alters the number or activation state of microglia in utero can profoundly affect neural development and affect behavioral outcomes.
Figures



Similar articles
-
Periventricular microglial cells interact with dividing precursor cells in the nonhuman primate and rodent prenatal cerebral cortex.J Comp Neurol. 2019 Jul 1;527(10):1598-1609. doi: 10.1002/cne.24604. Epub 2019 Jan 25. J Comp Neurol. 2019. PMID: 30552670 Free PMC article.
-
Endogenous microglia regulate development of embryonic cortical precursor cells.J Neurosci Res. 2011 Mar;89(3):286-98. doi: 10.1002/jnr.22533. Epub 2011 Jan 6. J Neurosci Res. 2011. PMID: 21259316
-
Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents.PLoS One. 2012;7(1):e30178. doi: 10.1371/journal.pone.0030178. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22272298 Free PMC article.
-
[CEREBRAL MICROGLIA AND MICROGLIAL MARKERS].Morfologiia. 2015;147(3):37-44. Morfologiia. 2015. PMID: 26390545 Review. Russian.
-
The interaction between microglia and neural stem/precursor cells.Brain Res Bull. 2014 Oct;109:32-8. doi: 10.1016/j.brainresbull.2014.09.005. Epub 2014 Sep 22. Brain Res Bull. 2014. PMID: 25245208 Review.
Cited by
-
Microglial trogocytosis and the complement system regulate axonal pruning in vivo.Elife. 2021 Mar 16;10:e62167. doi: 10.7554/eLife.62167. Elife. 2021. PMID: 33724186 Free PMC article.
-
Microglia Colonization Associated with Angiogenesis and Neural Cell Development.Adv Neurobiol. 2024;37:163-178. doi: 10.1007/978-3-031-55529-9_10. Adv Neurobiol. 2024. PMID: 39207692 Review.
-
Bioengineering Human Pluripotent Stem Cell-Derived Retinal Organoids and Optic Vesicle-Containing Brain Organoids for Ocular Diseases.Cells. 2022 Oct 30;11(21):3429. doi: 10.3390/cells11213429. Cells. 2022. PMID: 36359825 Free PMC article. Review.
-
Multifaceted microglia - key players in primary brain tumour heterogeneity.Nat Rev Neurol. 2021 Apr;17(4):243-259. doi: 10.1038/s41582-021-00463-2. Epub 2021 Mar 10. Nat Rev Neurol. 2021. PMID: 33692572 Review.
-
Fractalkine Receptor Deficiency Is Associated with Early Protection but Late Worsening of Outcome following Brain Trauma in Mice.J Neurotrauma. 2016 Jun 1;33(11):1060-72. doi: 10.1089/neu.2015.4041. Epub 2015 Sep 8. J Neurotrauma. 2016. PMID: 26180940 Free PMC article.
References
-
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. - PubMed
-
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. - PubMed
-
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. - PubMed
-
- Ashwell K. The distribution of microglia and cell death in the fetal rat forebrain. Brain Res Dev Brain Res. 1991;58:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
