Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells
- PMID: 23463011
- PMCID: PMC3615374
- DOI: 10.1038/ncomms2532
Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells
Abstract
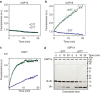
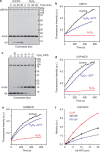
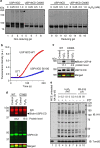
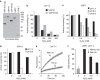
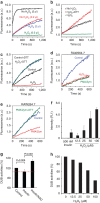
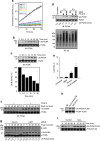
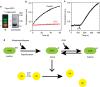
In eukaryotes, deubiquitinases (DUBs) remove ubiquitin conjugates from diverse substrates, altering their stabilities, localizations or activities. Here we show that many DUBs of the USP and UCH subfamilies can be reversibly inactivated upon oxidation by reactive oxygen species in vitro and in cells. Oxidation occurs preferentially on the catalytic cysteine, abrogating the isopeptide-cleaving activity without affecting these enzymes' affinity to ubiquitin. Sensitivity to oxidative inhibition is associated with DUB activation wherein the active site cysteine is converted to a deprotonated state prone to oxidation. We demonstrate that this redox regulation is essential for mono-ubiquitination of proliferating-cell nuclear antigen in response to oxidative DNA damage, which initiates a DNA damage-tolerance programme. These findings establish a novel mechanism of DUB regulation that may be integrated with other redox-dependent signalling circuits to govern cellular adaptation to oxidative stress, a process intimately linked to aging and cancer.
Figures
Similar articles
-
Deubiquitinases as a signaling target of oxidative stress.Cell Rep. 2012 Dec 27;2(6):1475-84. doi: 10.1016/j.celrep.2012.11.011. Epub 2012 Dec 6. Cell Rep. 2012. PMID: 23219552 Free PMC article.
-
Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines.Biochemistry. 2009 Feb 17;48(6):1399-409. doi: 10.1021/bi801973z. Biochemistry. 2009. PMID: 19166311
-
Regulation of A20 and other OTU deubiquitinases by reversible oxidation.Nat Commun. 2013;4:1569. doi: 10.1038/ncomms2567. Nat Commun. 2013. PMID: 23463012 Free PMC article.
-
Redox regulation of fertilisation and the spermatogenic process.Asian J Androl. 2011 May;13(3):420-3. doi: 10.1038/aja.2011.10. Epub 2011 Apr 4. Asian J Androl. 2011. PMID: 21460861 Free PMC article. Review.
-
Redox regulation of cysteine-dependent enzymes.J Anim Sci. 2010 Apr;88(4):1297-306. doi: 10.2527/jas.2009-2381. Epub 2009 Oct 9. J Anim Sci. 2010. PMID: 19820057 Review.
Cited by
-
A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity.Cold Spring Harb Perspect Biol. 2020 Jan 2;12(1):a036418. doi: 10.1101/cshperspect.a036418. Cold Spring Harb Perspect Biol. 2020. PMID: 31427375 Free PMC article. Review.
-
Human UFSP1 is an active protease that regulates UFM1 maturation and UFMylation.Cell Rep. 2022 Aug 2;40(5):111168. doi: 10.1016/j.celrep.2022.111168. Cell Rep. 2022. PMID: 35926457 Free PMC article.
-
USP1 deubiquitinase: cellular functions, regulatory mechanisms and emerging potential as target in cancer therapy.Mol Cancer. 2013 Aug 10;12:91. doi: 10.1186/1476-4598-12-91. Mol Cancer. 2013. PMID: 23937906 Free PMC article. Review.
-
Cellular mechanisms and physiological consequences of redox-dependent signalling.Nat Rev Mol Cell Biol. 2014 Jun;15(6):411-21. doi: 10.1038/nrm3801. Nat Rev Mol Cell Biol. 2014. PMID: 24854789 Review.
-
Ube2V2 Is a Rosetta Stone Bridging Redox and Ubiquitin Codes, Coordinating DNA Damage Responses.ACS Cent Sci. 2018 Feb 28;4(2):246-259. doi: 10.1021/acscentsci.7b00556. Epub 2018 Jan 17. ACS Cent Sci. 2018. PMID: 29532025 Free PMC article.
References
-
- Bergink S. & Jentsch S. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458, 461–467 (2009) . - PubMed
-
- Pickart C. M. & Fushman D. Polyubiquitin chains: polymeric protein signals. Curr. Opin. Chem. Biol. 8, 610–616 (2004) . - PubMed
-
- Wilkinson K. D. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 11, 1245–1256 (1997) . - PubMed
-
- Komander D., Clague M. J. & Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell. Biol. 10, 550–563 (2009) . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

