SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex
- PMID: 23461683
- PMCID: PMC3797451
- DOI: 10.1089/ars.2012.4713
SirT1 regulation of antioxidant genes is dependent on the formation of a FoxO3a/PGC-1α complex
Abstract
SirT1 is a class III histone deacetylase that has been implicated in metabolic and reactive oxygen species control. In the vasculature it has been shown to decrease endothelial superoxide production, prevent endothelial dysfunction and atherosclerosis. However, the mechanisms that mediate SirT1 antioxidant functions remain to be characterized. The transcription factor FoxO3a and the transcriptional coactivator peroxisome proliferator activated receptor γ-coactivator 1α (PGC-1α) have been shown to induce the expression of antioxidant genes and to be deacetylated by SirT1.
Aims: Here we investigated SirT1 regulation of antioxidant genes and the roles played by FoxO3a and PGC-1α in this regulation.
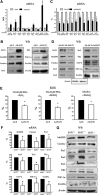
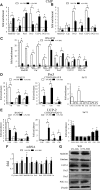
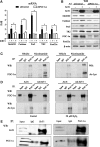
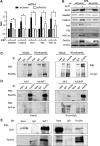
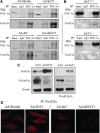
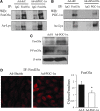
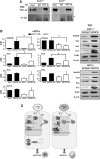
Results: We found that SirT1 regulates the expression of several antioxidant genes in bovine aortic endothelial cells, including Mn superoxide dismutase (MnSOD), catalase, peroxiredoxins 3 and 5 (Prx3, Prx5), thioredoxin 2 (Trx2), thioredoxin reductase 2 (TR2), and uncoupling protein 2 (UCP-2) and can be localized in the regulatory regions of these genes. We also found that knockdown of either FoxO3a or PGC-1α prevented the induction of antioxidant genes by SirT1 over-expression. Furthermore, SirT1 increased the formation of a FoxO3a/PGC-1α complex as determined by co-immunoprecipitation (IP) assays, concomitantly reducing H2O2-dependent FoxO3a and PGC-1α acetylation. Data showing that FoxO3a knockdown increases PGC-1α acetylation levels and vice versa, suggest that SirT1 activity on FoxO3a and PGC-1α may be dependent of the formation of a FoxO3a/PGC-1α complex.
Innovation: A unifying mechanism for SirT1 activities is suggested.
Conclusion: We show that SirT1 regulation of antioxidant genes in vascular endothelial cells depends on the formation of a FoxO3a/PGC-1α complex.
Figures
Similar articles
-
Peroxisome proliferator-activated receptor γ coactivator 1α and FoxO3A mediate chondroprotection by AMP-activated protein kinase.Arthritis Rheumatol. 2014 Nov;66(11):3073-82. doi: 10.1002/art.38791. Arthritis Rheumatol. 2014. PMID: 25047750 Free PMC article.
-
Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes.J Biol Chem. 2009 May 22;284(21):14476-84. doi: 10.1074/jbc.M807397200. Epub 2009 Mar 26. J Biol Chem. 2009. PMID: 19324885 Free PMC article.
-
Peroxisome proliferator-activated receptor γ coactivator-1α is a central negative regulator of vascular senescence.Arterioscler Thromb Vasc Biol. 2013 May;33(5):988-98. doi: 10.1161/ATVBAHA.112.301019. Epub 2013 Feb 21. Arterioscler Thromb Vasc Biol. 2013. PMID: 23430617 Free PMC article.
-
PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism.Oxid Med Cell Longev. 2020 Mar 9;2020:1452696. doi: 10.1155/2020/1452696. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32215168 Free PMC article. Review.
-
Deacetylation of PGC-1α by SIRT1: importance for skeletal muscle function and exercise-induced mitochondrial biogenesis.Appl Physiol Nutr Metab. 2011 Oct;36(5):589-97. doi: 10.1139/h11-070. Epub 2011 Sep 2. Appl Physiol Nutr Metab. 2011. PMID: 21888529 Review.
Cited by
-
Maternal dietary antioxidant supplementation regulates weaned piglets' adipose tissue transcriptome and morphology.PLoS One. 2024 Sep 12;19(9):e0310399. doi: 10.1371/journal.pone.0310399. eCollection 2024. PLoS One. 2024. PMID: 39264906 Free PMC article.
-
A remarkable age-related increase in SIRT1 protein expression against oxidative stress in elderly: SIRT1 gene variants and longevity in human.PLoS One. 2015 Mar 18;10(3):e0117954. doi: 10.1371/journal.pone.0117954. eCollection 2015. PLoS One. 2015. PMID: 25785999 Free PMC article. Clinical Trial.
-
Role of PGC-1α in Vascular Regulation: Implications for Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 Aug;36(8):1467-74. doi: 10.1161/ATVBAHA.116.307123. Epub 2016 Jun 16. Arterioscler Thromb Vasc Biol. 2016. PMID: 27312223 Free PMC article. Review.
-
Cystathionine γ-lyase S-sulfhydrates SIRT1 to attenuate myocardial death in isoprenaline-induced heart failure.Redox Rep. 2023 Dec;28(1):2174649. doi: 10.1080/13510002.2023.2174649. Redox Rep. 2023. PMID: 36757027 Free PMC article.
-
Redox signaling at the crossroads of human health and disease.MedComm (2020). 2022 Mar 31;3(2):e127. doi: 10.1002/mco2.127. eCollection 2022 Jun. MedComm (2020). 2022. PMID: 35386842 Free PMC article. Review.
References
-
- Alcendor RR. Gao S. Zhai P. Zablocki D. Holle E. Yu X. Tian B. Wagner T. Vatner SF. Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. - PubMed
-
- Amat R. Planavila A. Chen SL. Iglesias R. Giralt M. Villarroya F. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-gamma Co-activator-1alpha (PGC-1alpha) gene in skeletal muscle through the PGC-1alpha autoregulatory loop and interaction with MyoD. J Biol Chem. 2009;284:21872–21880. - PMC - PubMed
-
- Arden GB. Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7:291–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

