IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation
- PMID: 23460728
- PMCID: PMC3600911
- DOI: 10.1084/jem.20120572
IL-27 inhibits HIV-1 infection in human macrophages by down-regulating host factor SPTBN1 during monocyte to macrophage differentiation
Abstract
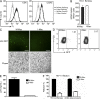
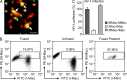
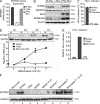
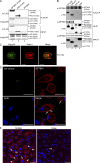
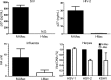
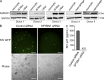
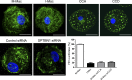
The susceptibility of macrophages to HIV-1 infection is modulated during monocyte differentiation. IL-27 is an anti-HIV cytokine that also modulates monocyte activation. In this study, we present new evidence that IL-27 promotes monocyte differentiation into macrophages that are nonpermissive for HIV-1 infection. Although IL-27 treatment does not affect expression of macrophage differentiation markers or macrophage biological functions, it confers HIV resistance by down-regulating spectrin β nonerythrocyte 1 (SPTBN1), a required host factor for HIV-1 infection. IL-27 down-regulates SPTBN1 through a TAK-1-mediated MAPK signaling pathway. Knockdown of SPTBN1 strongly inhibits HIV-1 infection of macrophages; conversely, overexpression of SPTBN1 markedly increases HIV susceptibility of IL-27-treated macrophages. Moreover, we demonstrate that SPTBN1 associates with HIV-1 gag proteins. Collectively, our results underscore the ability of IL-27 to protect macrophages from HIV-1 infection by down-regulating SPTBN1, thus indicating that SPTBN1 is an important host target to reduce HIV-1 replication in one major element of the viral reservoir.
Figures



Similar articles
-
Moderate restriction of macrophage-tropic human immunodeficiency virus type 1 by SAMHD1 in monocyte-derived macrophages.PLoS One. 2014 Mar 5;9(3):e90969. doi: 10.1371/journal.pone.0090969. eCollection 2014. PLoS One. 2014. PMID: 24599229 Free PMC article.
-
Interleukin-27-induced HIV-resistant dendritic cells suppress reveres transcription following virus entry in an SPTBN1, autophagy, and YB-1 independent manner.PLoS One. 2023 Nov 1;18(11):e0287829. doi: 10.1371/journal.pone.0287829. eCollection 2023. PLoS One. 2023. PMID: 37910521 Free PMC article.
-
Inhibition of human immunodeficiency virus replication in differentiating monocytes by interleukin 10 occurs in parallel with inhibition of cellular RNA expression.AIDS Res Hum Retroviruses. 1996 Sep 1;12(13):1237-45. doi: 10.1089/aid.1996.12.1237. AIDS Res Hum Retroviruses. 1996. PMID: 8870845
-
Regulation of chemokine/cytokine network during in vitro differentiation and HIV-1 infection of human monocytes: possible importance in the pathogenesis of AIDS.J Leukoc Biol. 2000 Sep;68(3):391-9. J Leukoc Biol. 2000. PMID: 10985256 Review.
-
Effect of GM-CSF on HIV-1 replication in monocytes/macrophages in vivo and in vitro: a review.Vet Immunol Immunopathol. 1998 May 15;63(1-2):111-21. doi: 10.1016/s0165-2427(98)00087-7. Vet Immunol Immunopathol. 1998. PMID: 9656446 Review.
Cited by
-
HIV-1 Treated Patients with Undetectable Viral Loads have Lower Levels of Innate Immune Responses via Cytosolic DNA Sensing Systems Compared with Healthy Uninfected Controls.J AIDS Clin Res. 2014 Jun;5(6):315. doi: 10.4172/2155-6113.1000315. J AIDS Clin Res. 2014. PMID: 26023356 Free PMC article.
-
TLR7 Ligation Inhibits TLR8 Responsiveness in IL-27-Primed Human THP-1 Monocytes and Macrophages.J Innate Immun. 2021;13(6):345-358. doi: 10.1159/000515738. Epub 2021 May 31. J Innate Immun. 2021. PMID: 34058746 Free PMC article.
-
IL-27 Expression and Responsiveness in Human Uterine Epithelial Cells and Fibroblasts In Vitro and the Role of Estradiol.J Interferon Cytokine Res. 2018 Mar;38(3):101-110. doi: 10.1089/jir.2017.0038. J Interferon Cytokine Res. 2018. PMID: 29565744 Free PMC article.
-
Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells.Immunity. 2014 Sep 18;41(3):493-502. doi: 10.1016/j.immuni.2014.08.014. Immunity. 2014. PMID: 25238099 Free PMC article.
-
IL-27 Modulates the Cytokine Secretion in the T Cell-Osteoclast Crosstalk During HIV Infection.Front Immunol. 2022 Apr 5;13:818677. doi: 10.3389/fimmu.2022.818677. eCollection 2022. Front Immunol. 2022. PMID: 35479090 Free PMC article.
References
-
- Andrei G., Fiten P., De Clercq E., Snoeck R., Opdenakker G. 2000. Monitoring drug resistance for herpesviruses. Methods Mol. Med. 24:151–169 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

