Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial
- PMID: 23460714
- PMCID: PMC3612594
- DOI: 10.1200/JCO.2012.45.4272
Phase III randomized, placebo-controlled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial
Abstract
Purpose: We hypothesized that the addition of gefitinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, to docetaxel would enhance therapeutic efficacy in squamous cell carcinoma of the head and neck (SCCHN).
Patients and methods: Patients with recurrent or metastatic SCCHN with Eastern Cooperative Oncology Group (ECOG) performance status of 2, or patients with ECOG performance status of 0 to 2 but were previously treated with chemotherapy, were randomly assigned to receive weekly docetaxel plus either placebo (arm A) or gefitinib 250 mg/d, orally (arm B) until disease progression. At the time of progression, patients in the placebo arm could receive single-agent gefitinib. EGFR, c-MET, and KRAS mutations and polymorphisms in drug metabolizing enzymes and transporters were evaluated by pyrosequencing.
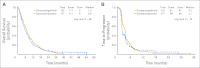
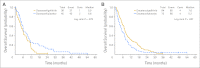
Results: Two hundred seventy patients were enrolled before the study was closed early at interim analysis (arm A, n = 136; arm B, n = 134). Median overall survival was 6.0 months in arm A versus 7.3 months in arm B (hazard ratio, 0.93; 95% CI, 0.72 to 1.21; P = .60). An unplanned subset analysis showed that gefitinib improved survival in patients younger than 65 years (median 7.6 v 5.2 months; P = .04). Also, there was a trend for improved survival in patients with c-MET wild-type (5.7 v 3.6 months; P = .09) regardless of treatment. Grade 3/4 toxicities were comparable between the two arms except that grade 3/4 diarrhea was more common with docetaxel/gefitinib. Of 18 eligible patients who received gefitinib after disease progression in arm A, one patient had a partial response.
Conclusion: The addition of gefitinib to docetaxel was well tolerated but did not improve outcomes in poor prognosis but otherwise unselected patients with SCCHN.
Trial registration: ClinicalTrials.gov NCT00088907.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Epidermal growth factor receptor targeting in head and neck cancer: have we been just skimming the surface?J Clin Oncol. 2013 Apr 10;31(11):1381-3. doi: 10.1200/JCO.2012.47.9220. Epub 2013 Mar 4. J Clin Oncol. 2013. PMID: 23460713 No abstract available.
Similar articles
-
Docetaxel and irinotecan in recurrent or metastatic head and neck cancer: a phase 2 trial of the Eastern Cooperative Oncology Group.Cancer. 2009 Oct 1;115(19):4504-13. doi: 10.1002/cncr.24528. Cancer. 2009. PMID: 19634157 Free PMC article. Clinical Trial.
-
Phase II trial of erlotinib and docetaxel in advanced and refractory hepatocellular and biliary cancers: Hoosier Oncology Group GI06-101.Oncologist. 2012;17(1):13. doi: 10.1634/theoncologist.2011-0253. Epub 2011 Dec 30. Oncologist. 2012. PMID: 22210086 Free PMC article. Clinical Trial.
-
A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN).Oral Oncol. 2013 Aug;49(8):835-41. doi: 10.1016/j.oraloncology.2013.04.010. Epub 2013 May 30. Oral Oncol. 2013. PMID: 23727257 Clinical Trial.
-
Gefitinib, Methotrexate and Methotrexate plus 5-Fluorouracil as palliative treatment in recurrent head and neck squamous cell carcinoma.Cancer Biol Ther. 2015;16(2):346-51. doi: 10.4161/15384047.2014.961881. Cancer Biol Ther. 2015. PMID: 25756517 Free PMC article. Clinical Trial.
-
Reasons for response differences seen in the V15-32, INTEREST and IPASS trials.Nat Rev Clin Oncol. 2009 May;6(5):287-94. doi: 10.1038/nrclinonc.2009.37. Nat Rev Clin Oncol. 2009. PMID: 19390555 Review.
Cited by
-
Emerging drugs for head and neck cancer.Expert Opin Emerg Drugs. 2015 Jun;20(2):313-29. doi: 10.1517/14728214.2015.1031653. Epub 2015 Mar 31. Expert Opin Emerg Drugs. 2015. PMID: 25826749 Free PMC article. Review.
-
Unraveling the molecular genetics of head and neck cancer through genome-wide approaches.Genes Dis. 2014 Sep;1(1):75-86. doi: 10.1016/j.gendis.2014.07.002. Genes Dis. 2014. PMID: 25642447 Free PMC article.
-
Biologic therapy in head and neck cancer: a road with hurdles.ISRN Oncol. 2012;2012:163752. doi: 10.5402/2012/163752. Epub 2012 Jun 13. ISRN Oncol. 2012. PMID: 22745915 Free PMC article.
-
The 4717C > G polymorphism in periplakin modulates sensitivity to EGFR inhibitors.Sci Rep. 2019 Feb 20;9(1):2357. doi: 10.1038/s41598-019-38742-0. Sci Rep. 2019. PMID: 30787334 Free PMC article.
-
Oropharyngeal squamous cell carcinoma treatment: current standards and future directions.Curr Opin Oncol. 2014 May;26(3):252-8. doi: 10.1097/CCO.0000000000000072. Curr Opin Oncol. 2014. PMID: 24626127 Free PMC article. Review.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Argiris A, Li Y, Forastiere A. Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer. 2004;101:2222–2229. - PubMed
-
- Stewart JS, Cohen EE, Licitra L, et al. Phase III study of gefitinib compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck [corrected] J Clin Oncol. 2009;27:1864–1871. - PubMed
-
- Hitt R, Amador ML, Quintela-Fandino M, et al. Weekly docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Cancer. 2006;106:106–111. - PubMed
-
- Guardiola E, Peyrade F, Chaigneau L, et al. Results of a randomised phase II study comparing docetaxel with methotrexate in patients with recurrent head and neck cancer. Eur J Cancer. 2004;40:2071–2076. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA66636/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA016116/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA16116/CA/NCI NIH HHS/United States
- U10 CA039229/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- CA39229/CA/NCI NIH HHS/United States
- CA21115/CA/NCI NIH HHS/United States
- U24 CA114737/CA/NCI NIH HHS/United States
- CA23318/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

