Characterization of printable cellular micro-fluidic channels for tissue engineering
- PMID: 23458889
- PMCID: PMC4281173
- DOI: 10.1088/1758-5082/5/2/025004
Characterization of printable cellular micro-fluidic channels for tissue engineering
Abstract
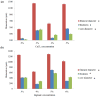
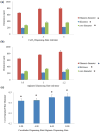
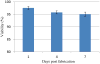
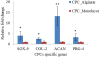
Tissue engineering has been a promising field of research, offering hope of bridging the gap between organ shortage and transplantation needs. However, building three-dimensional (3D) vascularized organs remains the main technological barrier to be overcome. One of the major challenges is the inclusion of a vascular network to support cell viability in terms of nutrients and oxygen perfusion. This paper introduces a new approach to the fabrication of vessel-like microfluidic channels that has the potential to be used in thick tissue or organ fabrication in the future. In this research, we investigate the manufacturability of printable micro-fluidic channels, where micro-fluidic channels support mechanical integrity as well as enable fluid transport in 3D. A pressure-assisted solid freeform fabrication platform is developed with a coaxial needle dispenser unit to print hollow hydrogel filaments. The dispensing rheology is studied, and effects of material properties on structural formation of hollow filaments are analyzed. Sample structures are printed through the developed computer-controlled system. In addition, cell viability and gene expression studies are presented in this paper. Cell viability shows that cartilage progenitor cells (CPCs) maintained their viability right after bioprinting and during prolonged in vitro culture. Real-time PCR analysis yielded a relatively higher expression of cartilage-specific genes in alginate hollow filament encapsulating CPCs, compared with monolayer cultured CPCs, which revealed that printable semi-permeable micro-fluidic channels provided an ideal environment for cell growth and function.
Figures
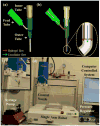





Similar articles
-
Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels.J Biomech Eng. 2013 Sep;135(9):91011. doi: 10.1115/1.4024575. J Biomech Eng. 2013. PMID: 23719889 Free PMC article.
-
Fabrication and characterization of gels with integrated channels using 3D printing with microfluidic nozzle for tissue engineering applications.Biomed Microdevices. 2016 Feb;18(1):17. doi: 10.1007/s10544-016-0042-6. Biomed Microdevices. 2016. PMID: 26842949
-
Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink.Adv Mater. 2016 Jan 27;28(4):677-84. doi: 10.1002/adma.201503310. Epub 2015 Nov 26. Adv Mater. 2016. PMID: 26606883 Free PMC article.
-
Bioprinting toward organ fabrication: challenges and future trends.IEEE Trans Biomed Eng. 2013 Mar;60(3):691-9. doi: 10.1109/TBME.2013.2243912. Epub 2013 Jan 30. IEEE Trans Biomed Eng. 2013. PMID: 23372076 Review.
-
Bioprinting and its applications in tissue engineering and regenerative medicine.Int J Biol Macromol. 2018 Feb;107(Pt A):261-275. doi: 10.1016/j.ijbiomac.2017.08.171. Epub 2017 Sep 21. Int J Biol Macromol. 2018. PMID: 28870749 Review.
Cited by
-
Three-dimensional bioprinting using self-assembling scalable scaffold-free "tissue strands" as a new bioink.Sci Rep. 2016 Jun 27;6:28714. doi: 10.1038/srep28714. Sci Rep. 2016. PMID: 27346373 Free PMC article.
-
Bioprinting functional tissues.Acta Biomater. 2019 Sep 1;95:32-49. doi: 10.1016/j.actbio.2019.01.009. Epub 2019 Jan 11. Acta Biomater. 2019. PMID: 30639351 Free PMC article. Review.
-
Alginate: Enhancement Strategies for Advanced Applications.Int J Mol Sci. 2022 Apr 19;23(9):4486. doi: 10.3390/ijms23094486. Int J Mol Sci. 2022. PMID: 35562876 Free PMC article. Review.
-
Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer's disease.Bioact Mater. 2021 Sep 23;11:192-205. doi: 10.1016/j.bioactmat.2021.09.023. eCollection 2022 May. Bioact Mater. 2021. PMID: 34938923 Free PMC article.
-
Extrusion-Based Bioprinting: Current Standards and Relevancy for Human-Sized Tissue Fabrication.Methods Mol Biol. 2020;2140:65-92. doi: 10.1007/978-1-0716-0520-2_5. Methods Mol Biol. 2020. PMID: 32207106
References
-
- Lanza R, Langer R, Vacanti J. Principles of Tissue Engineering. 3. Elsevier; 2007.
-
- Melchels FPW, Domingos MAN, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Progress in Polymer Science. 2012;37:1079–1104.
-
- Mondy WL, Cameron D, Timmermans JP, Clerk ND, Sasov A, Casteleyn C, Piegl L. 2009 Computer-aided design of microvasculature systems for use in vascular scaffold production. 2009;1:035002. - PubMed
-
- Lee W, Lee V, Polio S, Keegan P, Lee JH, Fischer K, Park JK, Yoo SS. On-demand three-dimensional freeform fabrication of multi-layered hydrogel scaffold with fluidic channels. Biotechnology and Bioengineering. 2010;105:1178–1186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
