ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue
- PMID: 23444362
- PMCID: PMC3912244
- DOI: 10.1242/dev.091844
ClearT: a detergent- and solvent-free clearing method for neuronal and non-neuronal tissue
Abstract
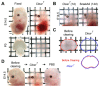
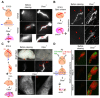
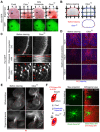
We describe a clearing method for enhanced visualization of cell morphology and connections in neuronal and non-neuronal tissue. Using Clear(T) or Clear(T2), which are composed of formamide or formamide/polyethylene glycol, respectively, embryos, whole mounts and thick brain sections can be rapidly cleared with minimal volume changes. Unlike other available clearing techniques, these methods do not use detergents or solvents, and thus preserve lipophilic dyes, fluorescent tracers and immunohistochemical labeling, as well as fluorescent-protein labeling.
Figures
Similar articles
-
RTF: a rapid and versatile tissue optical clearing method.Sci Rep. 2018 Jan 31;8(1):1964. doi: 10.1038/s41598-018-20306-3. Sci Rep. 2018. PMID: 29386656 Free PMC article.
-
Fluorescent double-labeling with carbocyanine neuronal tracing and immunohistochemistry using a cholesterol-specific detergent digitonin.J Neurosci Methods. 2008 Sep 15;174(1):71-81. doi: 10.1016/j.jneumeth.2008.07.003. Epub 2008 Jul 15. J Neurosci Methods. 2008. PMID: 18674563
-
A rapid approach to high-resolution fluorescence imaging in semi-thick brain slices.J Vis Exp. 2011 Jul 26;(53):2807. doi: 10.3791/2807. J Vis Exp. 2011. PMID: 21841756 Free PMC article.
-
Embryo slices and strips: guidance and adhesion assays in the avian embryo.Methods Cell Biol. 2008;87:97-113. doi: 10.1016/S0091-679X(08)00205-7. Methods Cell Biol. 2008. PMID: 18485293 Review. No abstract available.
-
A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue.Prog Histochem Cytochem. 2016 Aug;51(2):9-23. doi: 10.1016/j.proghi.2016.04.001. Epub 2016 Apr 14. Prog Histochem Cytochem. 2016. PMID: 27142295 Review.
Cited by
-
Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus.J Neurosci. 2013 Jul 10;33(28):11400-11. doi: 10.1523/JNEUROSCI.1374-13.2013. J Neurosci. 2013. PMID: 23843512 Free PMC article.
-
3D Visualization of the Temporal and Spatial Spread of Tau Pathology Reveals Extensive Sites of Tau Accumulation Associated with Neuronal Loss and Recognition Memory Deficit in Aged Tau Transgenic Mice.PLoS One. 2016 Jul 28;11(7):e0159463. doi: 10.1371/journal.pone.0159463. eCollection 2016. PLoS One. 2016. PMID: 27466814 Free PMC article.
-
Extracting structural and functional features of widely distributed biological circuits with single cell resolution via tissue clearing and delivery vectors.Curr Opin Biotechnol. 2016 Aug;40:193-207. doi: 10.1016/j.copbio.2016.03.012. Epub 2016 Jul 6. Curr Opin Biotechnol. 2016. PMID: 27393829 Free PMC article. Review.
-
On-chip clearing of arrays of 3-D cell cultures and micro-tissues.Biomicrofluidics. 2016 Jul 20;10(4):044107. doi: 10.1063/1.4959031. eCollection 2016 Jul. Biomicrofluidics. 2016. PMID: 27493703 Free PMC article.
-
Optimisation and validation of hydrogel-based brain tissue clearing shows uniform expansion across anatomical regions and spatial scales.Sci Rep. 2019 Aug 19;9(1):12084. doi: 10.1038/s41598-019-48460-2. Sci Rep. 2019. PMID: 31427619 Free PMC article.
References
-
- Bielle F., Marcos-Mondéjar P., Leyva-Díaz E., Lokmane L., Mire E., Mailhes C., Keita M., García N., Tessier-Lavigne M., Garel S., et al. (2011). Emergent growth cone responses to combinations of Slit1 and Netrin 1 in thalamocortical axon topography. Curr. Biol. 21, 1748–1755 - PubMed
-
- Dodt H. U., Leischner U., Schierloh A., Jährling N., Mauch C. P., Deininger K., Deussing J. M., Eder M., Zieglgänsberger W., Becker K. (2007). Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nat. Methods 4, 331–336 - PubMed
-
- Ertürk A., Mauch C. P., Hellal F., Förstner F., Keck T., Becker K., Jährling N., Steffens H., Richter M., Hübener M., et al. (2012). Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury. Nat. Med. 18, 166–171 - PubMed
-
- Feng G., Mellor R. H., Bernstein M., Keller-Peck C., Nguyen Q. T., Wallace M., Nerbonne J. M., Lichtman J. W., Sanes J. R. (2000). Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

