Safety of post-exposure rabies prophylaxis during pregnancy: a follow-up study from Guangzhou, China
- PMID: 23442589
- PMCID: PMC3667934
- DOI: 10.4161/hv.22377
Safety of post-exposure rabies prophylaxis during pregnancy: a follow-up study from Guangzhou, China
Abstract
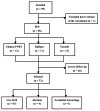
This study aimed to assess the safety profile of post-exposure prophylaxis (PEP) for rabies in pregnant women. All of the subjects received the Essen vaccination regimen. Systemic and local reactions were monitored within 72 hours following the immunization, and the subjects were followed until six months after delivery. No moderate or severe adverse effects occurred in any subject following the vaccination. Among the 72 subjects in this follow-up study, four had voluntary abortions, one subject had an accidental miscarriage, and the remaining 67 subjects delivered babies vaginally or by caesarean section. All of the infants exhibited normal development.The purified Vero cell rabies vaccine and the purified chick embryo cell vaccine were both safe for the PEP of pregnant women and did not interfere with the development of the fetuses or infants. Education is needed in China to stop pregnancy terminations due to concerns about rabies vaccination risk. :
Similar articles
-
Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study.Hum Vaccin Immunother. 2015;11(2):435-42. doi: 10.4161/21645515.2014.994460. Hum Vaccin Immunother. 2015. PMID: 25692350 Free PMC article. Clinical Trial.
-
A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults.Hum Vaccin Immunother. 2014;10(10):2805-12. doi: 10.4161/21645515.2014.972773. Hum Vaccin Immunother. 2014. PMID: 25483635 Free PMC article. Clinical Trial.
-
Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-8. doi: 10.1080/21645515.2017.1279770. Epub 2017 Jan 25. Hum Vaccin Immunother. 2017. PMID: 28121231 Free PMC article.
-
Post-exposure prophylaxis (PEP) for rabies with purified chick embryo cell vaccine: a systematic literature review and meta-analysis.Expert Rev Vaccines. 2018 Jun;17(6):525-545. doi: 10.1080/14760584.2018.1473765. Epub 2018 Jun 25. Expert Rev Vaccines. 2018. PMID: 29939085 Review.
-
[Post-exposure antirabies prophylaxis in pregnant women].Ginecol Obstet Mex. 1994 Jan;62:13-6. Ginecol Obstet Mex. 1994. PMID: 8168717 Review. Spanish.
Cited by
-
Rabies Vaccine Hesitancy and Deaths Among Pregnant and Breastfeeding Women - Vietnam, 2015-2016.MMWR Morb Mortal Wkly Rep. 2018 Mar 2;67(8):250-252. doi: 10.15585/mmwr.mm6708a4. MMWR Morb Mortal Wkly Rep. 2018. PMID: 29494566 Free PMC article.
-
Neutralizing Antibody Response after Intramuscular Purified Vero Cell Rabies Vaccination (PVRV) in Iranian Patients with Specific Medical Conditions.PLoS One. 2015 Oct 6;10(10):e0139171. doi: 10.1371/journal.pone.0139171. eCollection 2015. PLoS One. 2015. PMID: 26440665 Free PMC article. Clinical Trial.
-
Diagnostic difficulties in human rabies: A case report and review of the literature.Qatar Med J. 2017 Apr 21;2016(2):15. doi: 10.5339/qmj.2016.15. eCollection 2016. Qatar Med J. 2017. PMID: 28534007 Free PMC article.
-
Survival of a newborn from a pregnant woman with rabies infection.J Venom Anim Toxins Incl Trop Dis. 2016 Apr 2;22:14. doi: 10.1186/s40409-016-0068-5. eCollection 2016. J Venom Anim Toxins Incl Trop Dis. 2016. PMID: 27042172 Free PMC article.
-
A systematic review of ethical issues in vaccine studies involving pregnant women.Hum Vaccin Immunother. 2016 Aug 2;12(8):1952-1959. doi: 10.1080/21645515.2016.1186312. Epub 2016 May 31. Hum Vaccin Immunother. 2016. PMID: 27246403 Free PMC article. Review.
References
-
- Ashwathnarayana DH, Madhusudana SN, Sampath G, Sathpathy DM, Mankeshwar R, Ravish HH, et al. A comparative study on the safety and immunogenicity of Purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purifed vero cell rabies vaccine (PVRV, Verorab) Vaccine. 2009;28:148–51. doi: 10.1016/j.vaccine.2009.09.090. - DOI - PubMed
-
- WHO Expert Consultation on rabies. World Health Organ Tech Rep Ser 2005;931:1–88. back cover. - PubMed
-
- Centers for Disease Control and Prevention CDC American College of Obstetricians and Gynecologists ACOG: Vaccination during pregnancy. EPI Newsl. 1997;19:6. - PubMed
-
- Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, Lumbiganon P, Desimone JA, Baxter J, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev. 2006:D4903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous