Colloidal aggregation causes inhibition of G protein-coupled receptors
- PMID: 23437772
- PMCID: PMC3613083
- DOI: 10.1021/jm301749y
Colloidal aggregation causes inhibition of G protein-coupled receptors
Abstract
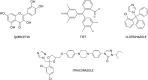
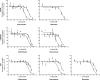
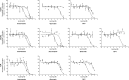
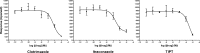
Colloidal aggregation is the dominant mechanism for artifactual inhibition of soluble proteins, and controls against it are now widely deployed. Conversely, investigating this mechanism for membrane-bound receptors has proven difficult. Here we investigate the activity of four well-characterized aggregators against three G protein-coupled receptors (GPCRs) recognizing peptide and protein ligands. Each of the aggregators was active at micromolar concentrations against the three GPCRs in cell-based assays. This activity could be attenuated by either centrifugation of the inhibitor stock solution or by addition of Tween-80 detergent. In the absence of agonist, the aggregators acted as inverse agonists, consistent with a direct receptor interaction. Meanwhile, several literature GPCR ligands that resemble aggregators themselves formed colloids, by both physical and enzymological tests. These observations suggest that some GPCRs may be artifactually antagonized by colloidal aggregates, an effect that merits the attention of investigators in this field.
Figures

Similar articles
-
Inverse agonists: tools to reveal ligand-specific conformations of G protein-coupled receptors.Sci STKE. 2004 Jan 5;2004(215):pe1. doi: 10.1126/stke.2152004pe1. Sci STKE. 2004. PMID: 14722344 Review.
-
An Aggregation Advisor for Ligand Discovery.J Med Chem. 2015 Sep 10;58(17):7076-87. doi: 10.1021/acs.jmedchem.5b01105. Epub 2015 Aug 28. J Med Chem. 2015. PMID: 26295373 Free PMC article.
-
Modulation of GPCR conformations by ligands, G-proteins, and arrestins.Ernst Schering Found Symp Proc. 2006;(2):211-28. Ernst Schering Found Symp Proc. 2006. PMID: 17703584 Review.
-
Biased signaling of G protein coupled receptors (GPCRs): Molecular determinants of GPCR/transducer selectivity and therapeutic potential.Pharmacol Ther. 2019 Aug;200:148-178. doi: 10.1016/j.pharmthera.2019.05.006. Epub 2019 May 8. Pharmacol Ther. 2019. PMID: 31075355 Review.
-
Conformational complexity of G-protein-coupled receptors.Trends Pharmacol Sci. 2007 Aug;28(8):397-406. doi: 10.1016/j.tips.2007.06.003. Epub 2007 Jul 13. Trends Pharmacol Sci. 2007. PMID: 17629961 Review.
Cited by
-
Identification of Novel Smoothened Ligands Using Structure-Based Docking.PLoS One. 2016 Aug 4;11(8):e0160365. doi: 10.1371/journal.pone.0160365. eCollection 2016. PLoS One. 2016. PMID: 27490099 Free PMC article.
-
A novel small molecule inhibitor of human Drp1.Sci Rep. 2022 Dec 13;12(1):21531. doi: 10.1038/s41598-022-25464-z. Sci Rep. 2022. PMID: 36513726 Free PMC article.
-
A New Spin on Antibody-Drug Conjugates: Trastuzumab-Fulvestrant Colloidal Drug Aggregates Target HER2-Positive Cells.ACS Appl Mater Interfaces. 2017 Apr 12;9(14):12195-12202. doi: 10.1021/acsami.6b15987. Epub 2017 Mar 28. ACS Appl Mater Interfaces. 2017. PMID: 28319364 Free PMC article.
-
Open Source Bayesian Models. 3. Composite Models for Prediction of Binned Responses.J Chem Inf Model. 2016 Feb 22;56(2):275-85. doi: 10.1021/acs.jcim.5b00555. Epub 2016 Jan 19. J Chem Inf Model. 2016. PMID: 26750305 Free PMC article.
-
A brief review of recent Charcot-Marie-Tooth research and priorities.F1000Res. 2015 Feb 26;4:53. doi: 10.12688/f1000research.6160.1. eCollection 2015. F1000Res. 2015. PMID: 25901280 Free PMC article. Review.
References
-
- Rishton G. M. Reactive compounds and in vitro false positives in HTS. Drug Discovery Today 1997, 2, 382–384.
-
- Walters W.; Namchuk M. Designing screens: how to make your hits a hit. Nat. Rev. Drug Discovery 2003, 2, 259–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

