Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium
- PMID: 23435541
- PMCID: PMC3629800
- DOI: 10.1124/jpet.112.201442
Attenuation by statins of membrane raft-redox signaling in coronary arterial endothelium
Abstract
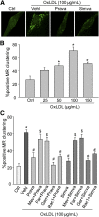
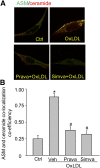
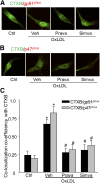
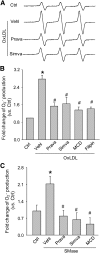
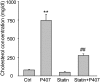
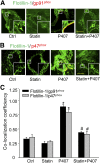
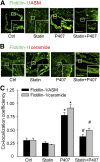
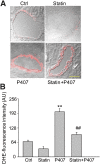
Membrane raft (MR)-redox signaling platforms associated with NADPH oxidase are involved in coronary endothelial dysfunction. Here, we studied whether statins interfere with the formation of MR-redox signaling platforms to protect the coronary arterial endothelium from oxidized low-density lipoprotein (OxLDL)-induced injury and from acute hypercholesterolemia. In cultured human coronary arterial endothelial cells, confocal microscopy detected the formation of an MRs clustering when they were exposed to OxLDL, and such MR platform formation was inhibited markedly by statins, including pravastatin and simvastatin. In these MR clusters, NADPH oxidase subunits gp91(phox) and p47(phox) were aggregated and were markedly blocked by both statins. In addition, colocalization of acid sphingomyelinase (ASM) and ceramide was induced by OxLDL, which was blocked by statins. Electron spin resonance spectrometry showed that OxLDL-induced superoxide (O2(.-)) production in the MR fractions was substantially reduced by statins. In coronary artery intima of mice with acute hypercholesterolemia, confocal microscopy revealed a colocalization of gp91(phox), p47(phox), ASM, or ceramide in MR clusters. Such colocalization was rarely observed in the arteries of normal mice or significantly reduced by pretreatment of hypercholesterolemic mice with statins. Furthermore, O2(.-) production in situ was 3-fold higher in the coronary arteries from hypercholesterolemic mice than in those from normal mice, and such increase was inhibited by statins. Our results indicate that blockade of MR-redox signaling platform formation in endothelial cell membrane may be another important therapeutic mechanism of statins in preventing endothelial injury and atherosclerosis and may be associated with their direct action on membrane cholesterol structure and function.
Figures
Similar articles
-
Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering.Antioxid Redox Signal. 2014 Mar 10;20(8):1198-215. doi: 10.1089/ars.2013.5430. Epub 2014 Jan 3. Antioxid Redox Signal. 2014. PMID: 24111702 Free PMC article. Review.
-
Membrane raft-lysosome redox signalling platforms in coronary endothelial dysfunction induced by adipokine visfatin.Cardiovasc Res. 2011 Feb 1;89(2):401-9. doi: 10.1093/cvr/cvq286. Epub 2010 Sep 7. Cardiovasc Res. 2011. PMID: 20823276 Free PMC article.
-
Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells.Antioxid Redox Signal. 2007 Jul;9(7):817-28. doi: 10.1089/ars.2007.1509. Antioxid Redox Signal. 2007. PMID: 17508908
-
Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction.Arterioscler Thromb Vasc Biol. 2008 Mar;28(3):485-90. doi: 10.1161/ATVBAHA.107.159772. Epub 2007 Dec 27. Arterioscler Thromb Vasc Biol. 2008. PMID: 18162606 Free PMC article.
-
Lipid raft redox signaling platforms in vascular dysfunction: features and mechanisms.Curr Atheroscler Rep. 2009 May;11(3):220-6. doi: 10.1007/s11883-009-0034-6. Curr Atheroscler Rep. 2009. PMID: 19361354 Review.
Cited by
-
Manipulating Oxidative Stress Following Ionizing Radiation.J Cell Signal. 2020;1(1):8-13. doi: 10.33696/signaling.1.003. J Cell Signal. 2020. PMID: 32550605 Free PMC article. No abstract available.
-
Membrane Remodeling as a Key Player of the Hepatotoxicity Induced by Co-Exposure to Benzo[a]pyrene and Ethanol of Obese Zebrafish Larvae.Biomolecules. 2018 May 14;8(2):26. doi: 10.3390/biom8020026. Biomolecules. 2018. PMID: 29757947 Free PMC article.
-
Protection by simvastatin on hyperglycemia-induced endothelial dysfunction through inhibiting NLRP3 inflammasomes.Oncotarget. 2017 Aug 24;8(53):91291-91305. doi: 10.18632/oncotarget.20443. eCollection 2017 Oct 31. Oncotarget. 2017. PMID: 29207644 Free PMC article.
-
Heritability and family-based GWAS analyses of the N-acyl ethanolamine and ceramide plasma lipidome.Hum Mol Genet. 2021 Apr 30;30(6):500-513. doi: 10.1093/hmg/ddab002. Hum Mol Genet. 2021. PMID: 33437986 Free PMC article.
-
Statins as regulators of redox state in the vascular endothelium: beyond lipid lowering.Antioxid Redox Signal. 2014 Mar 10;20(8):1198-215. doi: 10.1089/ars.2013.5430. Epub 2014 Jan 3. Antioxid Redox Signal. 2014. PMID: 24111702 Free PMC article. Review.
References
-
- (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389 - PubMed
-
- (1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 339:1349–1357 - PubMed
-
- Alvarez E, Rodiño-Janeiro BK, Ucieda-Somoza R, González-Juanatey JR. (2010) Pravastatin counteracts angiotensin II-induced upregulation and activation of NADPH oxidase at plasma membrane of human endothelial cells. J Cardiovasc Pharmacol 55:203–212 - PubMed
-
- Araki K, Masaki T, Katsuragi I, Kakuma T, Yoshimatsu H. (2008) Effects of pravastatin on obesity, diabetes, and adiponectin in diet-induced obese mice. Obesity (Silver Spring) 16:2068–2073 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

