Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction
- PMID: 23429874
- PMCID: PMC3633431
- DOI: 10.1152/japplphysiol.01506.2012
Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction
Abstract
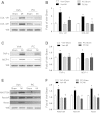
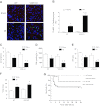
Accumulating evidence suggests that vitamin D deficiency plays a crucial role in heart failure. However, whether vitamin D signaling itself plays an important role in cardioprotection is poorly understood. In this study, we examined the mechanism of modulating vitamin D signaling on progression to heart failure after myocardial infarction (MI) in mice. Vitamin D signaling was activated by administration of paricalcitol (PC), an activated vitamin D analog. Wild-type (WT) mice underwent sham or MI surgery and then were treated with either vehicle or PC. Compared with vehicle group, PC attenuated development of heart failure after MI associated with decreases in biomarkers, apoptosis, inflammation, and fibrosis. There was also improvement of cardiac function with PC treatment after MI. Furthermore, vitamin D receptor (VDR) mRNA and protein levels were restored by PC treatment. Next, to explore whether defective vitamin D signaling exhibited deleterious responses after MI, WT and VDR knockout (KO) mice underwent sham or MI surgery and were analyzed 4 wk after MI. VDR KO mice displayed a significant decline in survival rate and cardiac function compared with WT mice after MI. VDR KO mice also demonstrated a significant increase in heart failure biomarkers, apoptosis, inflammation, and fibrosis. Vitamin D signaling promotes cardioprotection after MI through anti-inflammatory, antifibrotic and antiapoptotic mechanisms.
Figures




Similar articles
-
Ghrelin suppresses cardiac sympathetic activity and prevents early left ventricular remodeling in rats with myocardial infarction.Am J Physiol Heart Circ Physiol. 2008 Jan;294(1):H426-32. doi: 10.1152/ajpheart.00643.2007. Epub 2007 Nov 16. Am J Physiol Heart Circ Physiol. 2008. PMID: 18024547
-
DPP-4 inhibition has beneficial effects on the heart after myocardial infarction.J Mol Cell Cardiol. 2016 Feb;91:72-80. doi: 10.1016/j.yjmcc.2015.12.026. Epub 2015 Dec 29. J Mol Cell Cardiol. 2016. PMID: 26739213
-
Adiponectin determines farnesoid X receptor agonism-mediated cardioprotection against post-infarction remodelling and dysfunction.Cardiovasc Res. 2018 Aug 1;114(10):1335-1349. doi: 10.1093/cvr/cvy093. Cardiovasc Res. 2018. PMID: 29668847
-
Amplification of lipotoxic cardiomyopathy in the VDR gene knockout mouse.J Steroid Biochem Mol Biol. 2016 Nov;164:292-298. doi: 10.1016/j.jsbmb.2015.09.034. Epub 2015 Sep 30. J Steroid Biochem Mol Biol. 2016. PMID: 26429397 Review.
-
Vitamin D and the heart.Am J Physiol Regul Integr Comp Physiol. 2013 Nov 1;305(9):R969-77. doi: 10.1152/ajpregu.00322.2013. Epub 2013 Sep 11. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 24026071 Free PMC article. Review.
Cited by
-
The Role of Mannitol and Vitamin D in Ovarian Ischemia/Reperfusion Injury in Rats with Acute Abdominal.Curr Issues Mol Biol. 2024 Aug 15;46(8):8903-8913. doi: 10.3390/cimb46080526. Curr Issues Mol Biol. 2024. PMID: 39194743 Free PMC article.
-
Vitamin D Stimulates Cardiomyocyte Proliferation and Controls Organ Size and Regeneration in Zebrafish.Dev Cell. 2019 Mar 25;48(6):853-863.e5. doi: 10.1016/j.devcel.2019.01.001. Epub 2019 Jan 31. Dev Cell. 2019. PMID: 30713073 Free PMC article.
-
Vitamin D receptor activation: cardiovascular and renal implications.Kidney Int Suppl (2011). 2013 Dec;3(5):427-430. doi: 10.1038/kisup.2013.89. Kidney Int Suppl (2011). 2013. PMID: 25019025 Free PMC article. Review.
-
Role of Vitamin D in Cardiovascular Diseases.Endocrinol Metab Clin North Am. 2017 Dec;46(4):1039-1059. doi: 10.1016/j.ecl.2017.07.009. Epub 2017 Sep 29. Endocrinol Metab Clin North Am. 2017. PMID: 29080634 Free PMC article. Review.
-
Links between Vitamin D Deficiency and Cardiovascular Diseases.Biomed Res Int. 2015;2015:109275. doi: 10.1155/2015/109275. Epub 2015 Apr 27. Biomed Res Int. 2015. PMID: 26000280 Free PMC article. Review.
References
-
- Abbate A, Biondi-Zoccai GG, Bussani R, Dobrina A, Camilot D, Feroce F, Rossiello R, Baldi F, Silvestri F, Biasucci LM, Baldi A. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J Am Coll Cardiol 41: 753–760, 2003 - PubMed
-
- Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 - PubMed
-
- Baksi SN, Hughes MJ. Deficiency in dietary vitamin D, not calcium, alters noradrenergic responsiveness in rat atria in vitro. J Mol Cell Cardiol 18: 653–656, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

