Chronic cyclic bladder over distention up-regulates hypoxia dependent pathways
- PMID: 23429070
- PMCID: PMC4085185
- DOI: 10.1016/j.juro.2013.02.026
Chronic cyclic bladder over distention up-regulates hypoxia dependent pathways
Abstract
Purpose: Bladder over distention secondary to anatomical or functional obstruction can eventually lead to pathological changes, including decreased elasticity and contractile dysfunction. We hypothesized that chronic bladder distention in a murine model would activate hypoxia dependent signaling pathways despite intermittent relief of distention.
Materials and methods: Female C57Bl/6 mice were oophorectomized at age 5 to 6 weeks and underwent urethral catheterization and 90-minute bladder distention. Acute and chronic time points were evaluated. Bladder tissue was harvested for hematoxylin and eosin, and immunohistochemical staining with the hypoxia markers Glut-1 (EMD Millipore, Merck, Darmstadt, Germany) and Hypoxyprobe™-1. Bladder tissue was also harvested for real-time polymerase chain reaction and oxidative stress measurement. Hypoxia polymerase chain reaction arrays were done to determine changes in gene expression. Oxidative stress was measured using F2-IsoP. Functional bladder changes were evaluated using voided urine blots.
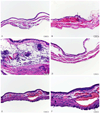
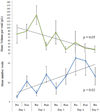
Results: After acute distention and 5 consecutive distentions, bladders showed marked inflammatory changes on hematoxylin and eosin staining, and evidence of tissue hypoxia on immunohistochemistry. Quantitative real-time polymerase chain reaction revealed up-regulation of hypoxia and oxidative stress related genes, including Hif1a, Arnt2, Ctgf, Gpx1 and Hmox1. Measurements of oxidative stress with F2-IsoP did not change. Voided urine blots before and after bladder distention showed marked changes with an overactive voiding pattern.
Conclusions: Chronic bladder distention is possible in the female mouse. It generates hypoxic injury, as characterized functionally by increased voiding patterns. This bladder injury model might more closely replicate bladder dysfunction in patients with poor bladder emptying due to neurological disease, including those noncompliant with intermittent catheterization.
Keywords: Arnt2; Ctgf; F2-IsoP; F2-isoprostane; Glut-1; Gpx1; HIF; Hmox1; ROS; anoxia; arylhydrocarbon receptor nuclear translocator 2; connective tissue growth factor; gene expression; glucose transporter 1; glutathione peroxidase 1; hemoxygenase 1; hypoxia-inducible factor; oxidative stress; pBOO; partial bladder outlet obstruction; qRT-PCR; quantitative real-time polymerase chain reaction; reactive oxygen species; urinary bladder; urinary bladder neck obstruction.
Copyright © 2013 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Effects of varying degrees of partial bladder outlet obstruction on urinary bladder function of rats: A novel link to inflammation, oxidative stress and hypoxia.Low Urin Tract Symptoms. 2019 Apr;11(2):O193-O201. doi: 10.1111/luts.12211. Epub 2017 Dec 28. Low Urin Tract Symptoms. 2019. PMID: 29282885
-
Modulation of the hypoxic response following partial bladder outlet obstruction.J Urol. 2012 Oct;188(4 Suppl):1549-54. doi: 10.1016/j.juro.2012.02.037. Epub 2012 Aug 19. J Urol. 2012. PMID: 22910264
-
The inflammatory cytokine IL-1β is involved in bladder remodeling after bladder outlet obstruction in mice.Neurourol Urodyn. 2016 Mar;35(3):377-81. doi: 10.1002/nau.22721. Epub 2015 Jan 3. Neurourol Urodyn. 2016. PMID: 25557558
-
Water avoidance stress results in an altered voiding phenotype in male mice.Neurourol Urodyn. 2012 Sep;31(7):1185-9. doi: 10.1002/nau.22207. Epub 2012 Mar 30. Neurourol Urodyn. 2012. PMID: 22473515
-
Review of Animal Models to Study Urinary Bladder Function.Biology (Basel). 2021 Dec 11;10(12):1316. doi: 10.3390/biology10121316. Biology (Basel). 2021. PMID: 34943231 Free PMC article. Review.
Cited by
-
HMOX1 silencing prevents doxorubicin-induced cardiomyocyte injury, mitochondrial dysfunction, and ferroptosis by downregulating CTGF.Gen Thorac Cardiovasc Surg. 2023 May;71(5):280-290. doi: 10.1007/s11748-022-01867-7. Epub 2022 Aug 25. Gen Thorac Cardiovasc Surg. 2023. PMID: 36008747
-
Inhibition of hypoxia-inducible factor-prolyl hydroxylation protects from cyclophosphamide-induced bladder injury and urinary dysfunction.Am J Physiol Renal Physiol. 2022 Jul 1;323(1):F81-F91. doi: 10.1152/ajprenal.00344.2021. Epub 2022 May 2. Am J Physiol Renal Physiol. 2022. PMID: 35499237 Free PMC article.
-
Bladder Dysfunction in Older Adults: The Botulinum Toxin Option.Drugs Aging. 2022 Jun;39(6):401-416. doi: 10.1007/s40266-022-00950-1. Epub 2022 Jun 13. Drugs Aging. 2022. PMID: 35696022 Review.
-
Inhibition of DNA methylation during chronic obstructive bladder disease (COBD) improves function, pathology and expression.Sci Rep. 2021 Aug 27;11(1):17307. doi: 10.1038/s41598-021-96155-4. Sci Rep. 2021. PMID: 34453065 Free PMC article.
-
A Review of Oxidative Stress and Urinary Dysfunction Caused by Bladder Outlet Obstruction and Treatments Using Antioxidants.Antioxidants (Basel). 2019 May 15;8(5):132. doi: 10.3390/antiox8050132. Antioxidants (Basel). 2019. PMID: 31096597 Free PMC article. Review.
References
-
- Drzewiecki BA, Anumanthan G, Penn HA, et al. Modulation of the hypoxic response following partial bladder outlet obstruction. J Urol. 2012;188:1549. - PubMed
-
- Woo LL, Tanaka ST, Anumanthan G, et al. Mesenchymal stem cell recruitment and improved bladder function after bladder outlet obstruction: preliminary data. J Urol. 2011;185:1132. - PubMed
-
- Greenland JE, Brading AF. The effect of bladder outflow obstruction on detrusor blood flow changes during the voiding cycle in conscious pigs. J Urol. 2001;165:245. - PubMed
-
- Brading AF. Alterations in the physiological properties of urinary bladder smooth muscle caused by bladder emptying against an obstruction. Scand J Urol Nephrol Suppl. 1997;184:51. - PubMed
-
- Zderic SA, Butler S, Sliwoski J. Hypoxia Inducible Factor – the Tipping Point for Bladder Decompensation Following Partial Outlet Obstruction. Presented at the American Academy of Pediatrics - Section on Urology; October 15th, 2011; Boston, MA.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

