GRL-0519, a novel oxatricyclic ligand-containing nonpeptidic HIV-1 protease inhibitor (PI), potently suppresses replication of a wide spectrum of multi-PI-resistant HIV-1 variants in vitro
- PMID: 23403426
- PMCID: PMC3632947
- DOI: 10.1128/AAC.02189-12
GRL-0519, a novel oxatricyclic ligand-containing nonpeptidic HIV-1 protease inhibitor (PI), potently suppresses replication of a wide spectrum of multi-PI-resistant HIV-1 variants in vitro
Abstract
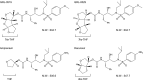
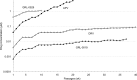
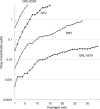
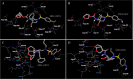
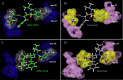
We report that GRL-0519, a novel nonpeptidic human immunodeficiency virus type 1 (HIV-1) protease inhibitor (PI) containing tris-tetrahydrofuranylurethane (tris-THF) and a sulfonamide isostere, is highly potent against laboratory HIV-1 strains and primary clinical isolates (50% effective concentration [EC50], 0.0005 to 0.0007 μM) with minimal cytotoxicity (50% cytotoxic concentration [CC50], 44.6 μM). GRL-0519 blocked the infectivity and replication of HIV-1NL4-3 variants selected by up to a 5 μM concentration of ritonavir, lopinavir, or atazanavir (EC50, 0.0028 to 0.0033 μM). GRL-0519 was also potent against multi-PI-resistant clinical HIV-1 variants isolated from patients who no longer responded to existing antiviral regimens after long-term antiretroviral therapy, highly darunavir (DRV)-resistant variants, and HIV-2ROD. The development of resistance against GRL-0519 was substantially delayed compared to other PIs, including amprenavir (APV) and DRV. The effects of nonspecific binding of human serum proteins on GRL-0519's antiviral activity were insignificant. Our analysis of the crystal structures of GRL-0519 (3OK9) and DRV (2IEN) with protease suggested that the tris-THF moiety, compared to the bis-THF moiety present in DRV, has greater water-mediated polar interactions with key active-site residues of protease and that the tris-THF moiety and paramethoxy group effectively fill the S2 and S2' binding pockets, respectively, of the protease. The present data demonstrate that GRL-0519 has highly favorable features as a potential therapeutic agent for treating patients infected with wild-type and/or multi-PI-resistant variants and that the tris-THF moiety is critical for strong binding of GRL-0519 to the HIV protease substrate binding site and appears to be responsible for its favorable antiretroviral characteristics.
Figures


Similar articles
-
GRL-04810 and GRL-05010, difluoride-containing nonpeptidic HIV-1 protease inhibitors (PIs) that inhibit the replication of multi-PI-resistant HIV-1 in vitro and possess favorable lipophilicity that may allow blood-brain barrier penetration.Antimicrob Agents Chemother. 2013 Dec;57(12):6110-21. doi: 10.1128/AAC.01420-13. Epub 2013 Sep 30. Antimicrob Agents Chemother. 2013. PMID: 24080647 Free PMC article.
-
A Modified P1 Moiety Enhances In Vitro Antiviral Activity against Various Multidrug-Resistant HIV-1 Variants and In Vitro Central Nervous System Penetration Properties of a Novel Nonpeptidic Protease Inhibitor, GRL-10413.Antimicrob Agents Chemother. 2016 Nov 21;60(12):7046-7059. doi: 10.1128/AAC.01428-16. Print 2016 Dec. Antimicrob Agents Chemother. 2016. PMID: 27620483 Free PMC article.
-
A novel tricyclic ligand-containing nonpeptidic HIV-1 protease inhibitor, GRL-0739, effectively inhibits the replication of multidrug-resistant HIV-1 variants and has a desirable central nervous system penetration property in vitro.Antimicrob Agents Chemother. 2015 May;59(5):2625-35. doi: 10.1128/AAC.04757-14. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691652 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Resilience to resistance of HIV-1 protease inhibitors: profile of darunavir.AIDS Rev. 2008 Jul-Sep;10(3):131-42. AIDS Rev. 2008. PMID: 18820715 Free PMC article. Review.
Cited by
-
Highly resistant HIV-1 proteases and strategies for their inhibition.Future Med Chem. 2015;7(8):1023-38. doi: 10.4155/fmc.15.44. Future Med Chem. 2015. PMID: 26062399 Free PMC article. Review.
-
Structural studies of antiviral inhibitor with HIV-1 protease bearing drug resistant substitutions of V32I, I47V and V82I.Biochem Biophys Res Commun. 2019 Jun 30;514(3):974-978. doi: 10.1016/j.bbrc.2019.05.064. Epub 2019 May 12. Biochem Biophys Res Commun. 2019. PMID: 31092330 Free PMC article.
-
Dimerization of HIV-1 protease occurs through two steps relating to the mechanism of protease dimerization inhibition by darunavir.Proc Natl Acad Sci U S A. 2014 Aug 19;111(33):12234-9. doi: 10.1073/pnas.1400027111. Epub 2014 Aug 4. Proc Natl Acad Sci U S A. 2014. PMID: 25092296 Free PMC article.
-
GRL-04810 and GRL-05010, difluoride-containing nonpeptidic HIV-1 protease inhibitors (PIs) that inhibit the replication of multi-PI-resistant HIV-1 in vitro and possess favorable lipophilicity that may allow blood-brain barrier penetration.Antimicrob Agents Chemother. 2013 Dec;57(12):6110-21. doi: 10.1128/AAC.01420-13. Epub 2013 Sep 30. Antimicrob Agents Chemother. 2013. PMID: 24080647 Free PMC article.
-
Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS.J Med Chem. 2016 Jun 9;59(11):5172-208. doi: 10.1021/acs.jmedchem.5b01697. Epub 2016 Jan 22. J Med Chem. 2016. PMID: 26799988 Free PMC article. Review.
References
-
- Edmonds A, Yotebieng M, Lusiama J, Matumona Y, Kitetele F, Napravnik S, Cole SR, Van Rie A, Behets F. 2011. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med. 8:e1001044 doi:10.1371/journal.pmed.1001044 - DOI - PMC - PubMed
-
- Lohse N, Hansen AB, Gerstoft J, Obel N. 2007. Improved survival in HIV-infected persons: consequences and perspectives. J. Antimicrob. Chemother. 60:461–463 - PubMed
-
- Mitsuya H, Maeda K, Das D, Ghosh AK. 2008. Development of protease inhibitors and the fight with drug-resistant HIV-1 variants. Adv. Pharmacol. 56:169–197 - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA. 2006. The survival benefits of AIDS treatment in the United States. J. Infect. Dis. 194:11–19 - PubMed
-
- De Clercq E. 2002. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 1:13–25 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

