Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy
- PMID: 23401527
- PMCID: PMC3607024
- DOI: 10.1073/pnas.1300398110
Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy
Abstract
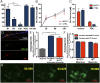
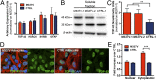
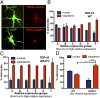
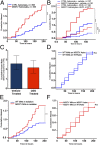
Glial proliferation and activation are associated with disease progression in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar dementia. In this study, we describe a unique platform to address the question of cell autonomy in transactive response DNA-binding protein (TDP-43) proteinopathies. We generated functional astroglia from human induced pluripotent stem cells carrying an ALS-causing TDP-43 mutation and show that mutant astrocytes exhibit increased levels of TDP-43, subcellular mislocalization of TDP-43, and decreased cell survival. We then performed coculture experiments to evaluate the effects of M337V astrocytes on the survival of wild-type and M337V TDP-43 motor neurons, showing that mutant TDP-43 astrocytes do not adversely affect survival of cocultured neurons. These observations reveal a significant and previously unrecognized glial cell-autonomous pathological phenotype associated with a pathogenic mutation in TDP-43 and show that TDP-43 proteinopathies do not display an astrocyte non-cell-autonomous component in cell culture, as previously described for SOD1 ALS. This study highlights the utility of induced pluripotent stem cell-based in vitro disease models to investigate mechanisms of disease in ALS and other TDP-43 proteinopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Glia as primary drivers of neuropathology in TDP-43 proteinopathies.Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4439-40. doi: 10.1073/pnas.1301608110. Epub 2013 Mar 7. Proc Natl Acad Sci U S A. 2013. PMID: 23471990 Free PMC article. No abstract available.
-
Unpicking neurodegeneration in a dish with human pluripotent stem cells: one cell type at a time.Cell Cycle. 2013 Aug 1;12(15):2339-40. doi: 10.4161/cc.25705. Epub 2013 Jul 11. Cell Cycle. 2013. PMID: 23856579 Free PMC article. No abstract available.
Similar articles
-
Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis.Brain. 2020 Feb 1;143(2):430-440. doi: 10.1093/brain/awz419. Brain. 2020. PMID: 32040555 Free PMC article.
-
Mutant induced pluripotent stem cell lines recapitulate aspects of TDP-43 proteinopathies and reveal cell-specific vulnerability.Proc Natl Acad Sci U S A. 2012 Apr 10;109(15):5803-8. doi: 10.1073/pnas.1202922109. Epub 2012 Mar 26. Proc Natl Acad Sci U S A. 2012. PMID: 22451909 Free PMC article.
-
Altered astrocytic expression of TDP-43 does not influence motor neuron survival.Exp Neurol. 2013 Dec;250:250-9. doi: 10.1016/j.expneurol.2013.10.004. Epub 2013 Oct 9. Exp Neurol. 2013. PMID: 24120466
-
Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies.Neuropathology. 2010 Apr;30(2):103-12. doi: 10.1111/j.1440-1789.2009.01091.x. Epub 2010 Jan 25. Neuropathology. 2010. PMID: 20102519 Free PMC article. Review.
-
Glial TDP-43 and TDP-43 induced glial pathology, focus on neurodegenerative proteinopathy syndromes.Glia. 2022 Feb;70(2):239-255. doi: 10.1002/glia.24096. Epub 2021 Sep 24. Glia. 2022. PMID: 34558120 Free PMC article. Review.
Cited by
-
Stress granule formation helps to mitigate neurodegeneration.Nucleic Acids Res. 2024 Sep 9;52(16):9745-9759. doi: 10.1093/nar/gkae655. Nucleic Acids Res. 2024. PMID: 39106168 Free PMC article.
-
Direct conversion of fibroblasts into functional astrocytes by defined transcription factors.Stem Cell Reports. 2015 Jan 13;4(1):25-36. doi: 10.1016/j.stemcr.2014.12.002. Epub 2014 Dec 31. Stem Cell Reports. 2015. PMID: 25556566 Free PMC article.
-
Downstream Effects of Mutations in SOD1 and TARDBP Converge on Gene Expression Impairment in Patient-Derived Motor Neurons.Int J Mol Sci. 2022 Aug 25;23(17):9652. doi: 10.3390/ijms23179652. Int J Mol Sci. 2022. PMID: 36077049 Free PMC article.
-
A cellular star atlas: using astrocytes from human pluripotent stem cells for disease studies.Front Cell Neurosci. 2013 Mar 14;7:25. doi: 10.3389/fncel.2013.00025. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23503583 Free PMC article.
-
Transactive response DNA-binding protein-43 proteinopathy in oligodendrocytes revealed using an induced pluripotent stem cell model.Brain Commun. 2021 Oct 26;3(4):fcab255. doi: 10.1093/braincomms/fcab255. eCollection 2021. Brain Commun. 2021. PMID: 35350711 Free PMC article.
References
-
- Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. - PubMed
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. - PubMed
-
- Marchetto MCN, et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell. 2008;3(6):649–657. - PubMed
-
- Ince PG, et al. Molecular pathology and genetic advances in amyotrophic lateral sclerosis: An emerging molecular pathway and the significance of glial pathology. Acta Neuropathol. 2011;122(6):657–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

