RNA-programmed genome editing in human cells
- PMID: 23386978
- PMCID: PMC3557905
- DOI: 10.7554/eLife.00471
RNA-programmed genome editing in human cells
Abstract
Type II CRISPR immune systems in bacteria use a dual RNA-guided DNA endonuclease, Cas9, to cleave foreign DNA at specific sites. We show here that Cas9 assembles with hybrid guide RNAs in human cells and can induce the formation of double-strand DNA breaks (DSBs) at a site complementary to the guide RNA sequence in genomic DNA. This cleavage activity requires both Cas9 and the complementary binding of the guide RNA. Experiments using extracts from transfected cells show that RNA expression and/or assembly into Cas9 is the limiting factor for Cas9-mediated DNA cleavage. In addition, we find that extension of the RNA sequence at the 3' end enhances DNA targeting activity in vivo. These results show that RNA-programmed genome editing is a facile strategy for introducing site-specific genetic changes in human cells.DOI:http://dx.doi.org/10.7554/eLife.00471.001.
Keywords: Cas9; Human; endonuclease; genome editing.
Conflict of interest statement
MJ, Founder of Caribou Biosciences and member of its scientific advisory board. Has filed a provisional patent related to this work.
JD, Founder of Caribou Biosciences and member of its scientific advisory board. Has filed a provisional patent related to this work.
The other authors declare that no competing interests exist.
Figures
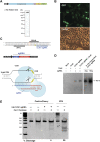

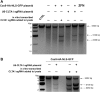
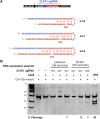
Comment in
- doi: 10.7554/eLife.00563
Similar articles
-
CRISPR/Cas9 for genome editing: progress, implications and challenges.Hum Mol Genet. 2014 Sep 15;23(R1):R40-6. doi: 10.1093/hmg/ddu125. Epub 2014 Mar 20. Hum Mol Genet. 2014. PMID: 24651067 Review.
-
Gene Therapy with CRISPR/Cas9 Coming to Age for HIV Cure.AIDS Rev. 2017 Oct-Dec;19(3):167-172. AIDS Rev. 2017. PMID: 29019352
-
CRISPR/Cas9-mediated genome editing of Epstein-Barr virus in human cells.J Gen Virol. 2015 Mar;96(Pt 3):626-636. doi: 10.1099/jgv.0.000012. Epub 2014 Dec 12. J Gen Virol. 2015. PMID: 25502645
-
RNA-dependent DNA endonuclease Cas9 of the CRISPR system: Holy Grail of genome editing?Trends Microbiol. 2013 Nov;21(11):562-7. doi: 10.1016/j.tim.2013.09.001. Epub 2013 Oct 1. Trends Microbiol. 2013. PMID: 24095303
-
[Application of CRISPR-Cas9 genome editing for constructing animal models of human diseases].Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016 Aug;33(4):559-63. doi: 10.3760/cma.j.issn.1003-9406.2016.04.030. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016. PMID: 27455021 Review. Chinese.
Cited by
-
The NIH Somatic Cell Genome Editing program.Nature. 2021 Apr;592(7853):195-204. doi: 10.1038/s41586-021-03191-1. Epub 2021 Apr 7. Nature. 2021. PMID: 33828315 Free PMC article. Review.
-
Different Effects of sgRNA Length on CRISPR-mediated Gene Knockout Efficiency.Sci Rep. 2016 Jun 24;6:28566. doi: 10.1038/srep28566. Sci Rep. 2016. PMID: 27338021 Free PMC article.
-
Precision Modulation of Neurodegenerative Disease-Related Gene Expression in Human iPSC-Derived Neurons.Sci Rep. 2016 Jun 24;6:28420. doi: 10.1038/srep28420. Sci Rep. 2016. PMID: 27341390 Free PMC article.
-
Genome editing of human pluripotent stem cells to generate human cellular disease models.Dis Model Mech. 2013 Jul;6(4):896-904. doi: 10.1242/dmm.012054. Epub 2013 Jun 10. Dis Model Mech. 2013. PMID: 23751357 Free PMC article. Review.
-
A gap-free genome assembly of Chlamydomonas reinhardtii and detection of translocations induced by CRISPR-mediated mutagenesis.Plant Commun. 2023 Mar 13;4(2):100493. doi: 10.1016/j.xplc.2022.100493. Epub 2022 Nov 17. Plant Commun. 2023. PMID: 36397679 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

