ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43
- PMID: 23382207
- PMCID: PMC3581922
- DOI: 10.1073/pnas.1222809110
ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43
Abstract
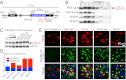
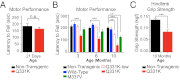
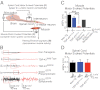
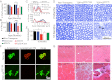
Transactivating response region DNA binding protein (TDP-43) is the major protein component of ubiquitinated inclusions found in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) with ubiquitinated inclusions. Two ALS-causing mutants (TDP-43(Q331K) and TDP-43(M337V)), but not wild-type human TDP-43, are shown here to provoke age-dependent, mutant-dependent, progressive motor axon degeneration and motor neuron death when expressed in mice at levels and in a cell type-selective pattern similar to endogenous TDP-43. Mutant TDP-43-dependent degeneration of lower motor neurons occurs without: (i) loss of TDP-43 from the corresponding nuclei, (ii) accumulation of TDP-43 aggregates, and (iii) accumulation of insoluble TDP-43. Computational analysis using splicing-sensitive microarrays demonstrates alterations of endogenous TDP-43-dependent alternative splicing events conferred by both human wild-type and mutant TDP-43(Q331K), but with high levels of mutant TDP-43 preferentially enhancing exon exclusion of some target pre-mRNAs affecting genes involved in neurological transmission and function. Comparison with splicing alterations following TDP-43 depletion demonstrates that TDP-43(Q331K) enhances normal TDP-43 splicing function for some RNA targets but loss-of-function for others. Thus, adult-onset motor neuron disease does not require aggregation or loss of nuclear TDP-43, with ALS-linked mutants producing loss and gain of splicing function of selected RNA targets at an early disease stage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Wild type human TDP-43 potentiates ALS-linked mutant TDP-43 driven progressive motor and cortical neuron degeneration with pathological features of ALS.Acta Neuropathol Commun. 2015 Jun 25;3:36. doi: 10.1186/s40478-015-0212-4. Acta Neuropathol Commun. 2015. PMID: 26108367 Free PMC article.
-
Quantification of the Relative Contributions of Loss-of-function and Gain-of-function Mechanisms in TAR DNA-binding Protein 43 (TDP-43) Proteinopathies.J Biol Chem. 2016 Sep 9;291(37):19437-48. doi: 10.1074/jbc.M116.737726. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445339 Free PMC article.
-
Mutant TDP-43 within motor neurons drives disease onset but not progression in amyotrophic lateral sclerosis.Acta Neuropathol. 2017 Jun;133(6):907-922. doi: 10.1007/s00401-017-1698-6. Epub 2017 Mar 29. Acta Neuropathol. 2017. PMID: 28357566 Free PMC article.
-
Understanding the role of TDP-43 and FUS/TLS in ALS and beyond.Curr Opin Neurobiol. 2011 Dec;21(6):904-19. doi: 10.1016/j.conb.2011.05.029. Epub 2011 Aug 2. Curr Opin Neurobiol. 2011. PMID: 21813273 Free PMC article. Review.
-
[Clinical and pathological spectrum of TDP-43 associated ALS].Rinsho Shinkeigaku. 2010 Nov;50(11):940-2. doi: 10.5692/clinicalneurol.50.940. Rinsho Shinkeigaku. 2010. PMID: 21921519 Review. Japanese.
Cited by
-
Stress granules as crucibles of ALS pathogenesis.J Cell Biol. 2013 Apr 29;201(3):361-72. doi: 10.1083/jcb.201302044. J Cell Biol. 2013. PMID: 23629963 Free PMC article. Review.
-
Wild type human TDP-43 potentiates ALS-linked mutant TDP-43 driven progressive motor and cortical neuron degeneration with pathological features of ALS.Acta Neuropathol Commun. 2015 Jun 25;3:36. doi: 10.1186/s40478-015-0212-4. Acta Neuropathol Commun. 2015. PMID: 26108367 Free PMC article.
-
Proteotoxicity and Neurodegenerative Diseases.Int J Mol Sci. 2020 Aug 6;21(16):5646. doi: 10.3390/ijms21165646. Int J Mol Sci. 2020. PMID: 32781742 Free PMC article. Review.
-
Traumatic injury compromises nucleocytoplasmic transport and leads to TDP-43 pathology.Elife. 2021 May 26;10:e67587. doi: 10.7554/eLife.67587. Elife. 2021. PMID: 34060470 Free PMC article.
-
Excessive nucleic acid R-loops induce mitochondria-dependent epithelial cell necroptosis and drive spontaneous intestinal inflammation.Proc Natl Acad Sci U S A. 2024 Jan 2;121(1):e2307395120. doi: 10.1073/pnas.2307395120. Epub 2023 Dec 29. Proc Natl Acad Sci U S A. 2024. PMID: 38157451 Free PMC article.
References
-
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59(7):1077–1079. - PubMed
-
- Neumann M, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. - PubMed
-
- Arai T, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351(3):602–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AG 000216/AG/NIA NIH HHS/United States
- NS075216/NS/NINDS NIH HHS/United States
- NS075449/NS/NINDS NIH HHS/United States
- RC1 NS069144/NS/NINDS NIH HHS/United States
- K99 NS075216/NS/NINDS NIH HHS/United States
- MC_G1000733/MRC_/Medical Research Council/United Kingdom
- T32 GM008666/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- G0300329/MRC_/Medical Research Council/United Kingdom
- G0900688/MRC_/Medical Research Council/United Kingdom
- 089701/WT_/Wellcome Trust/United Kingdom
- HG004659/HG/NHGRI NIH HHS/United States
- R01 HG004659/HG/NHGRI NIH HHS/United States
- T32 AG000216/AG/NIA NIH HHS/United States
- NS069144/NS/NINDS NIH HHS/United States
- R01 NS075449/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

