DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation
- PMID: 23378590
- PMCID: PMC3552502
- DOI: 10.1101/cshperspect.a012559
DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation
Abstract
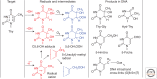
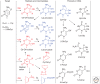
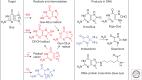
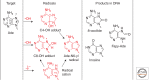
Emphasis has been placed in this article dedicated to DNA damage on recent aspects of the formation and measurement of oxidatively generated damage in cellular DNA in order to provide a comprehensive and updated survey. This includes single pyrimidine and purine base lesions, intrastrand cross-links, purine 5',8-cyclonucleosides, DNA-protein adducts and interstrand cross-links formed by the reactions of either the nucleobases or the 2-deoxyribose moiety with the hydroxyl radical, one-electron oxidants, singlet oxygen, and hypochlorous acid. In addition, recent information concerning the mechanisms of formation, individual measurement, and repair-rate assessment of bipyrimidine photoproducts in isolated cells and human skin upon exposure to UVB radiation, UVA photons, or solar simulated light is critically reviewed.
Figures
Similar articles
-
Oxidatively generated base damage to cellular DNA by hydroxyl radical and one-electron oxidants: similarities and differences.Arch Biochem Biophys. 2014 Sep 1;557:47-54. doi: 10.1016/j.abb.2014.05.001. Epub 2014 May 10. Arch Biochem Biophys. 2014. PMID: 24820329
-
Formation and repair of oxidatively generated damage in cellular DNA.Free Radic Biol Med. 2017 Jun;107:13-34. doi: 10.1016/j.freeradbiomed.2016.12.049. Epub 2017 Jan 2. Free Radic Biol Med. 2017. PMID: 28057600 Free PMC article. Review.
-
Contribution of oxidation reactions to photo-induced damage to cellular DNA.Photochem Photobiol. 2024 Sep-Oct;100(5):1157-1185. doi: 10.1111/php.13990. Epub 2024 Jul 5. Photochem Photobiol. 2024. PMID: 38970297 Review.
-
Oxidatively generated damage to cellular DNA by UVB and UVA radiation.Photochem Photobiol. 2015 Jan-Feb;91(1):140-55. doi: 10.1111/php.12368. Epub 2014 Nov 27. Photochem Photobiol. 2015. PMID: 25327445 Review.
-
One-electron oxidation reactions of purine and pyrimidine bases in cellular DNA.Int J Radiat Biol. 2014 Jun;90(6):423-32. doi: 10.3109/09553002.2013.877176. Epub 2014 Apr 3. Int J Radiat Biol. 2014. PMID: 24369822 Free PMC article. Review.
Cited by
-
Regulation of Nuclear Mechanics and the Impact on DNA Damage.Int J Mol Sci. 2021 Mar 20;22(6):3178. doi: 10.3390/ijms22063178. Int J Mol Sci. 2021. PMID: 33804722 Free PMC article. Review.
-
(Chemical) Roles of HOCl in Rheumatic Diseases.Antioxidants (Basel). 2024 Jul 29;13(8):921. doi: 10.3390/antiox13080921. Antioxidants (Basel). 2024. PMID: 39199167 Free PMC article. Review.
-
Comprehensive Temporal Protein Dynamics during Postirradiation Recovery in Deinococcus radiodurans.Oxid Med Cell Longev. 2022 Nov 11;2022:1622829. doi: 10.1155/2022/1622829. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36411759 Free PMC article.
-
Transcriptional Profiling of Porcine Blastocysts Produced In Vitro in a Chemically Defined Culture Medium.Animals (Basel). 2021 May 14;11(5):1414. doi: 10.3390/ani11051414. Animals (Basel). 2021. PMID: 34069238 Free PMC article.
-
Elevated Inactivation Efficacy of a Pulsed UVC Light-Emitting Diode System for Foodborne Pathogens on Selective Media and Food Surfaces.Appl Environ Microbiol. 2018 Oct 1;84(20):e01340-18. doi: 10.1128/AEM.01340-18. Print 2018 Oct 15. Appl Environ Microbiol. 2018. PMID: 30097449 Free PMC article.
References
-
- Aida M, Nishimura S 1987. An ab initio molecular orbital study on the characteristics of 8-hydroxyguanine. Mut Res Lett 192: 83–89 - PubMed
-
- Asgatay S, Martinez A, Coantic-Castex S, Harakat D, Philippe C, Douki T, Clivio P 2010. UV-induced TA photoproducts: Formation and hydrolysis in double-stranded DNA. J Am Chem Soc 132: 10260–10261 - PubMed
-
- Azqueta A, Shaposhnikov S, Collins AR 2009. DNA oxidation: Investigating its key role in mutagenesis with the comet assay. Mutat Res 674: 101–108 - PubMed
-
- Badouard C, Masuda M, Nishino H, Cadet J, Favier A, Ravanat JL 2005. Detection of chlorinated DNA and RNA nucleosides by HPLC coupled to tandem mass spectrometry as potential biomarkers of inflammation. J Chromatogr B 827: 26–31 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
