Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation
- PMID: 23370291
- PMCID: PMC3899169
- DOI: 10.4161/hv.23800
Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation
Abstract
Objective: We aimed to compare the potential for inducing HIV production and the effect on T-cell activation of potent HDAC inhibitors undergoing clinical investigation.
Design: In vitro study
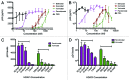
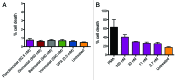
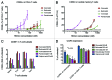
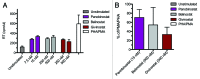
Results: The various HDAC inhibitors displayed significant potency differences in stimulating HIV-1 expression from the latently infected cell lines with panobinostat>givinostat ≈belinostat>vorinostat>valproic acid. Panobinostat was significantly more potent than all other HDAC inhibitors and induced virus production even in the very low concentration range 8-31 nM. The proportion of primary T-cells expressing the early activation marker CD69 increased moderately in all HDAC inhibitor-treated cells compared with untreated cells. Finally, proof was obtained that panobinostat, givinostat and belinostat induce virus production in latently infected primary cells at therapeutic concentrations with panobinostat being the most potent stimulator.
Methods: The latently infected cell lines ACH2 and U1 were treated with the HDAC inhibitors panobinostat, givinostat, belinostat, vorinostat and valproic acid. Viral induction was estimated by p24 production. Peripheral blood mononuclear cells from uninfected donors were treated with the HDAC inhibitors and the expression of activation markers on T-cell phenotypes was measured using flow cytometry. Finally, the ability of givinostat, belinostat and panobinostat to reactivate latent HIV-1 expression in primary T-cells was investigated employing a CCL19-induced latent primary CD4+ T cell infection model.
Conclusion: At therapeutic concentrations panobinostat stimulate HIV-1 expression in latently infected cells with greater potency than other HDAC inhibitors undergoing clinical investigation. These findings warrant further investigation and panobinostat is now being advanced into clinical testing against latent HIV infection.
Keywords: HIV; HIV cure; HIV eradication; histone deacetylase inhibitors.
Figures
Similar articles
-
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection.Retrovirology. 2016 May 21;13(1):36. doi: 10.1186/s12977-016-0268-7. Retrovirology. 2016. PMID: 27206407 Free PMC article.
-
Short Communication: The Broad-Spectrum Histone Deacetylase Inhibitors Vorinostat and Panobinostat Activate Latent HIV in CD4(+) T Cells In Part Through Phosphorylation of the T-Loop of the CDK9 Subunit of P-TEFb.AIDS Res Hum Retroviruses. 2016 Feb;32(2):169-73. doi: 10.1089/AID.2015.0347. AIDS Res Hum Retroviruses. 2016. PMID: 26727990 Free PMC article.
-
The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events.J Virol. 2014 Sep;88(18):10803-12. doi: 10.1128/JVI.00320-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008921 Free PMC article.
-
Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir.Mol Med. 2011 May-Jun;17(5-6):466-72. doi: 10.2119/molmed.2011.00076. Epub 2011 Mar 15. Mol Med. 2011. PMID: 21424110 Free PMC article. Review.
-
Reactivation of latent HIV by histone deacetylase inhibitors.Trends Microbiol. 2013 Jun;21(6):277-85. doi: 10.1016/j.tim.2013.02.005. Epub 2013 Mar 18. Trends Microbiol. 2013. PMID: 23517573 Free PMC article. Review.
Cited by
-
Multi-dose Romidepsin Reactivates Replication Competent SIV in Post-antiretroviral Rhesus Macaque Controllers.PLoS Pathog. 2016 Sep 15;12(9):e1005879. doi: 10.1371/journal.ppat.1005879. eCollection 2016 Sep. PLoS Pathog. 2016. PMID: 27632364 Free PMC article.
-
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection.Retrovirology. 2016 May 21;13(1):36. doi: 10.1186/s12977-016-0268-7. Retrovirology. 2016. PMID: 27206407 Free PMC article.
-
Selection of epigenetically privileged HIV-1 proviruses during treatment with panobinostat and interferon-α2a.Cell. 2024 Feb 29;187(5):1238-1254.e14. doi: 10.1016/j.cell.2024.01.037. Epub 2024 Feb 17. Cell. 2024. PMID: 38367616 Free PMC article. Clinical Trial.
-
Selective miRNA Modulation Fails to Activate HIV Replication in In Vitro Latency Models.Mol Ther Nucleic Acids. 2019 Sep 6;17:323-336. doi: 10.1016/j.omtn.2019.06.006. Epub 2019 Jun 20. Mol Ther Nucleic Acids. 2019. PMID: 31288207 Free PMC article.
-
Epigenetic Modulation of CD8⁺ T Cell Function in Lentivirus Infections: A Review.Viruses. 2018 Apr 28;10(5):227. doi: 10.3390/v10050227. Viruses. 2018. PMID: 29710792 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
