A role for the membrane in regulating Chlamydomonas flagellar length
- PMID: 23359798
- PMCID: PMC3554728
- DOI: 10.1371/journal.pone.0053366
A role for the membrane in regulating Chlamydomonas flagellar length
Abstract
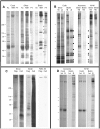
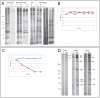
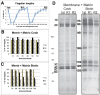
Flagellar assembly requires coordination between the assembly of axonemal proteins and the assembly of the flagellar membrane and membrane proteins. Fully grown steady-state Chlamydomonas flagella release flagellar vesicles from their tips and failure to resupply membrane should affect flagellar length. To study vesicle release, plasma and flagellar membrane surface proteins were vectorially pulse-labeled and flagella and vesicles were analyzed for biotinylated proteins. Based on the quantity of biotinylated proteins in purified vesicles, steady-state flagella appeared to shed a minimum of 16% of their surface membrane per hour, equivalent to a complete flagellar membrane being released every 6 hrs or less. Brefeldin-A destroyed Chlamydomonas Golgi, inhibited the secretory pathway, inhibited flagellar regeneration, and induced full-length flagella to disassemble within 6 hrs, consistent with flagellar disassembly being induced by a failure to resupply membrane. In contrast to membrane lipids, a pool of biotinylatable membrane proteins was identified that was sufficient to resupply flagella as they released vesicles for 6 hrs in the absence of protein synthesis and to support one and nearly two regenerations of flagella following amputation. These studies reveal the importance of the secretory pathway to assemble and maintain full-length flagella.
Conflict of interest statement
Figures

Similar articles
-
Polarity of flagellar assembly in Chlamydomonas.J Cell Biol. 1992 Dec;119(6):1605-11. doi: 10.1083/jcb.119.6.1605. J Cell Biol. 1992. PMID: 1281816 Free PMC article.
-
Flagellar elongation and shortening in Chlamydomonas. IV. Effects of flagellar detachment, regeneration, and resorption on the induction of flagellar protein synthesis.J Cell Biol. 1978 Jul;78(1):8-27. doi: 10.1083/jcb.78.1.8. J Cell Biol. 1978. PMID: 149796 Free PMC article.
-
Synthesis, transport, and utilization of specific flagellar proteins during flagellar regeneration in Chlamydomonas.J Cell Biol. 1982 Jun;93(3):615-31. doi: 10.1083/jcb.93.3.615. J Cell Biol. 1982. PMID: 7118994 Free PMC article.
-
Flagellar microtubule dynamics in Chlamydomonas: cytochalasin D induces periods of microtubule shortening and elongation; and colchicine induces disassembly of the distal, but not proximal, half of the flagellum.J Cell Biol. 1992 Jun;117(6):1289-98. doi: 10.1083/jcb.117.6.1289. J Cell Biol. 1992. PMID: 1607390 Free PMC article.
-
Directed movements of ciliary and flagellar membrane components: a review.Biol Cell. 1992;76(3):291-301. doi: 10.1016/0248-4900(92)90431-y. Biol Cell. 1992. PMID: 1305476 Review.
Cited by
-
The Biology of Ciliary Dynamics.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a027904. doi: 10.1101/cshperspect.a027904. Cold Spring Harb Perspect Biol. 2017. PMID: 28062565 Free PMC article. Review.
-
Transport and barrier mechanisms that regulate ciliary compartmentalization and ciliopathies.Nat Rev Nephrol. 2024 Feb;20(2):83-100. doi: 10.1038/s41581-023-00773-2. Epub 2023 Oct 23. Nat Rev Nephrol. 2024. PMID: 37872350 Review.
-
Ciliary Extracellular Vesicles: Txt Msg Organelles.Cell Mol Neurobiol. 2016 Apr;36(3):449-57. doi: 10.1007/s10571-016-0345-4. Epub 2016 Mar 17. Cell Mol Neurobiol. 2016. PMID: 26983828 Free PMC article. Review.
-
Initial ciliary assembly in Chlamydomonas requires Arp2/3 complex-dependent endocytosis.Mol Biol Cell. 2023 Apr 1;34(4):ar24. doi: 10.1091/mbc.E22-09-0443. Epub 2023 Feb 8. Mol Biol Cell. 2023. PMID: 36753382 Free PMC article.
-
Seriously cilia: A tiny organelle illuminates evolution, disease, and intercellular communication.Dev Cell. 2023 Aug 7;58(15):1333-1349. doi: 10.1016/j.devcel.2023.06.013. Epub 2023 Jul 24. Dev Cell. 2023. PMID: 37490910 Free PMC article. Review.
References
-
- Jekely G, Arendt G (2006) Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 28: 191–198. - PubMed
-
- Dentler WL (2009) Microtubule-membrane interactions in Chlamydomonas flagella. The Chlamydomonas Sourcebook, 2nd edition. G.B. Witman, ed. Academic Press, NY, 283–301.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

