Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development
- PMID: 23358245
- PMCID: PMC3563690
- DOI: 10.1083/jcb.201206028
Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development
Abstract
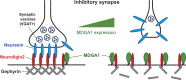
Rare variants in MDGAs (MAM domain-containing glycosylphosphatidylinositol anchors), including multiple protein-truncating deletions, are linked to autism and schizophrenia, but the function of these genes is poorly understood. Here, we show that MDGA1 and MDGA2 bound to neuroligin-2 inhibitory synapse-organizing protein, also implicated in neurodevelopmental disorders. MDGA1 inhibited the synapse-promoting activity of neuroligin-2, without altering neuroligin-2 surface trafficking, by inhibiting interaction of neuroligin-2 with neurexin. MDGA binding and suppression of synaptogenic activity was selective for neuroligin-2 and not neuroligin-1 excitatory synapse organizer. Overexpression of MDGA1 in cultured rat hippocampal neurons reduced inhibitory synapse density without altering excitatory synapse density. Furthermore, RNAi-mediated knockdown of MDGA1 selectively increased inhibitory but not excitatory synapse density. These results identify MDGA1 as one of few identified negative regulators of synapse development with a unique selectivity for inhibitory synapses. These results also place MDGAs in the neurexin-neuroligin synaptic pathway implicated in neurodevelopmental disorders and support the idea that an imbalance between inhibitory and excitatory synapses may contribute to these disorders.
Figures
Similar articles
-
Structural Insights into Modulation of Neurexin-Neuroligin Trans-synaptic Adhesion by MDGA1/Neuroligin-2 Complex.Neuron. 2017 Jun 21;94(6):1121-1131.e6. doi: 10.1016/j.neuron.2017.05.034. Neuron. 2017. PMID: 28641111
-
MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):336-41. doi: 10.1073/pnas.1219987110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248271 Free PMC article.
-
Molecular Mechanism of MDGA1: Regulation of Neuroligin 2:Neurexin Trans-synaptic Bridges.Neuron. 2017 Jun 21;94(6):1132-1141.e4. doi: 10.1016/j.neuron.2017.06.009. Neuron. 2017. PMID: 28641112 Free PMC article.
-
Pumping the brakes: suppression of synapse development by MDGA-neuroligin interactions.Curr Opin Neurobiol. 2019 Aug;57:71-80. doi: 10.1016/j.conb.2019.01.002. Epub 2019 Feb 14. Curr Opin Neurobiol. 2019. PMID: 30771697 Review.
-
Neuroligin-2 as a central organizer of inhibitory synapses in health and disease.Sci Signal. 2020 Dec 22;13(663):eabd8379. doi: 10.1126/scisignal.abd8379. Sci Signal. 2020. PMID: 33443230 Review.
Cited by
-
The Role of the Eukaryotic Translation Initiation Factor 4E (eIF4E) in Neuropsychiatric Disorders.Front Genet. 2018 Nov 23;9:561. doi: 10.3389/fgene.2018.00561. eCollection 2018. Front Genet. 2018. PMID: 30532767 Free PMC article. Review.
-
Functional mosaic organization of neuroligins in neuronal circuits.Cell Mol Life Sci. 2020 Aug;77(16):3117-3127. doi: 10.1007/s00018-020-03478-y. Epub 2020 Feb 20. Cell Mol Life Sci. 2020. PMID: 32077971 Free PMC article. Review.
-
Dynamic Control of Synaptic Adhesion and Organizing Molecules in Synaptic Plasticity.Neural Plast. 2017;2017:6526151. doi: 10.1155/2017/6526151. Epub 2017 Jan 31. Neural Plast. 2017. PMID: 28255461 Free PMC article. Review.
-
Increased Excitatory Synaptic Transmission of Dentate Granule Neurons in Mice Lacking PSD-95-Interacting Adhesion Molecule Neph2/Kirrel3 during the Early Postnatal Period.Front Mol Neurosci. 2017 Mar 22;10:81. doi: 10.3389/fnmol.2017.00081. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28381988 Free PMC article.
-
In vivo clonal overexpression of neuroligin 3 and neuroligin 2 in neurons of the rat cerebral cortex: Differential effects on GABAergic synapses and neuronal migration.J Comp Neurol. 2015 Jun 15;523(9):1359-78. doi: 10.1002/cne.23740. Epub 2015 Apr 8. J Comp Neurol. 2015. PMID: 25565602 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

