Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging
- PMID: 23354332
- PMCID: PMC4286370
- DOI: 10.1038/nn.3324
Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging
Abstract
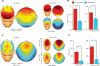
Aging has independently been associated with regional brain atrophy, reduced slow wave activity (SWA) during non-rapid eye movement (NREM) sleep and impaired long-term retention of episodic memories. However, whether the interaction of these factors represents a neuropatholgical pathway associated with cognitive decline in later life remains unknown. We found that age-related medial prefrontal cortex (mPFC) gray-matter atrophy was associated with reduced NREM SWA in older adults, the extent to which statistically mediated the impairment of overnight sleep-dependent memory retention. Moreover, this memory impairment was further associated with persistent hippocampal activation and reduced task-related hippocampal-prefrontal cortex functional connectivity, potentially representing impoverished hippocampal-neocortical memory transformation. Together, these data support a model in which age-related mPFC atrophy diminishes SWA, the functional consequence of which is impaired long-term memory. Such findings suggest that sleep disruption in the elderly, mediated by structural brain changes, represents a contributing factor to age-related cognitive decline in later life.
Figures

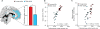
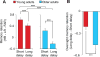
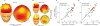
Similar articles
-
β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation.Nat Neurosci. 2015 Jul;18(7):1051-7. doi: 10.1038/nn.4035. Epub 2015 Jun 1. Nat Neurosci. 2015. PMID: 26030850 Free PMC article.
-
Memory quality modulates the effect of aging on memory consolidation during sleep: Reduced maintenance but intact gain.Neuroimage. 2020 Apr 1;209:116490. doi: 10.1016/j.neuroimage.2019.116490. Epub 2019 Dec 25. Neuroimage. 2020. PMID: 31883456 Free PMC article.
-
Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting.Neuron. 2018 Jan 3;97(1):221-230.e4. doi: 10.1016/j.neuron.2017.11.020. Epub 2017 Dec 14. Neuron. 2018. PMID: 29249289 Free PMC article.
-
Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states.Neurobiol Learn Mem. 2019 Apr;160:11-20. doi: 10.1016/j.nlm.2018.01.002. Epub 2018 Jan 10. Neurobiol Learn Mem. 2019. PMID: 29331447 Free PMC article. Review.
-
Oscillations and hippocampal-prefrontal synchrony.Curr Opin Neurobiol. 2011 Jun;21(3):467-74. doi: 10.1016/j.conb.2011.04.006. Epub 2011 May 14. Curr Opin Neurobiol. 2011. PMID: 21571522 Free PMC article. Review.
Cited by
-
Emerging co-morbidities of obstructive sleep apnea: cognition, kidney disease, and cancer.J Thorac Dis. 2016 Sep;8(9):E901-E917. doi: 10.21037/jtd.2016.09.23. J Thorac Dis. 2016. PMID: 27747026 Free PMC article. Review.
-
Multimodal neuroimaging correlates of spectral power in NREM sleep delta sub-bands in cognitively unimpaired older adults.Sleep. 2024 Apr 12;47(4):zsae012. doi: 10.1093/sleep/zsae012. Sleep. 2024. PMID: 38227830 Free PMC article. Clinical Trial.
-
Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation.Physiol Rev. 2021 Oct 1;101(4):1487-1559. doi: 10.1152/physrev.00022.2020. Epub 2021 Mar 26. Physiol Rev. 2021. PMID: 33769101 Free PMC article. Review.
-
The Impact of Sleep on Neurocognition and Functioning in Schizophrenia-Is It Time to Wake-Up?J Psychiatr Brain Sci. 2022;7:e220001. doi: 10.20900/jpbs.20220001. Epub 2022 Jan 25. J Psychiatr Brain Sci. 2022. PMID: 35224206 Free PMC article.
-
Sleep Deprivation Disrupts Acquisition of Contextual Fear Extinction by Affecting Circadian Oscillation of Hippocampal-Infralimbic proBDNF.eNeuro. 2019 Oct 17;6(5):ENEURO.0165-19.2019. doi: 10.1523/ENEURO.0165-19.2019. Print 2019 Sep/Oct. eNeuro. 2019. PMID: 31585927 Free PMC article.
References
-
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. - PubMed
-
- Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. - PubMed
-
- Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiology of aging. 1989;10:677–682. - PubMed
-
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Jama. 2000;284:861–868. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

