Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis
- PMID: 23352663
- PMCID: PMC3563842
- DOI: 10.1016/j.celrep.2012.12.017
Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis
Abstract
Mammary epithelial stem cells are vital to tissue expansion and remodeling during various phases of postnatal mammary development. Basal mammary epithelial cells are enriched in Wnt-responsive cells and can reconstitute cleared mammary fat pads upon transplantation into mice. Lgr5 is a Wnt-regulated target gene and was identified as a major stem cell marker in the small intestine, colon, stomach, and hair follicle, as well as in kidney nephrons. Here, we demonstrate the outstanding regenerative potential of a rare population of Lgr5-expressing (Lgr5(+)) mammary epithelial cells (MECs). We found that Lgr5(+) cells reside within the basal population, are superior to other basal cells in regenerating functional mammary glands (MGs), are exceptionally efficient in reconstituting MGs from single cells, and exhibit regenerative capacity in serial transplantations. Loss-of-function and depletion experiments of Lgr5(+) cells from transplanted MECs or from pubertal MGs revealed that these cells are not only sufficient but also necessary for postnatal mammary organogenesis.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

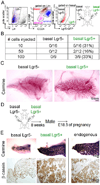
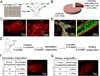

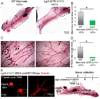
Similar articles
-
Developmental stage-specific contribution of LGR5(+) cells to basal and luminal epithelial lineages in the postnatal mammary gland.J Pathol. 2012 Nov;228(3):300-9. doi: 10.1002/path.4096. J Pathol. 2012. PMID: 22926799
-
Establishing estrogen-responsive mouse mammary organoids from single Lgr5+ cells.Cell Signal. 2017 Jan;29:41-51. doi: 10.1016/j.cellsig.2016.08.001. Epub 2016 Aug 7. Cell Signal. 2017. PMID: 27511963 Free PMC article.
-
Evidence for a multipotent mammary progenitor with pregnancy-specific activity.Breast Cancer Res. 2013;15(4):R65. doi: 10.1186/bcr3459. Breast Cancer Res. 2013. PMID: 23947835 Free PMC article.
-
Reprogramming non-mammary and cancer cells in the developing mouse mammary gland.Semin Cell Dev Biol. 2012 Jul;23(5):591-8. doi: 10.1016/j.semcdb.2012.03.007. Epub 2012 Mar 10. Semin Cell Dev Biol. 2012. PMID: 22430755 Free PMC article. Review.
-
[Characterization of stem cells from the murine adult mammary gland].Med Sci (Paris). 2007 Dec;23(12):1125-31. doi: 10.1051/medsci/200723121125. Med Sci (Paris). 2007. PMID: 18154715 Review. French.
Cited by
-
Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium.Breast Cancer Res. 2013 Apr 29;15(2):R36. doi: 10.1186/bcr3419. Breast Cancer Res. 2013. PMID: 23621987 Free PMC article.
-
Microvesicle-mediated Wnt/β-Catenin Signaling Promotes Interspecies Mammary Stem/Progenitor Cell Growth.J Biol Chem. 2016 Nov 18;291(47):24390-24405. doi: 10.1074/jbc.M116.726117. Epub 2016 Oct 12. J Biol Chem. 2016. PMID: 27733685 Free PMC article.
-
The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells?Cell Stem Cell. 2015 Mar 5;16(3):225-38. doi: 10.1016/j.stem.2015.02.015. Cell Stem Cell. 2015. PMID: 25748930 Free PMC article. Review.
-
Unraveling Heterogeneity in Epithelial Cell Fates of the Mammary Gland and Breast Cancer.Cancers (Basel). 2019 Sep 24;11(10):1423. doi: 10.3390/cancers11101423. Cancers (Basel). 2019. PMID: 31554261 Free PMC article. Review.
-
The role of R-spondin proteins in cancer biology.Oncogene. 2021 Nov;40(47):6469-6478. doi: 10.1038/s41388-021-02059-y. Epub 2021 Oct 18. Oncogene. 2021. PMID: 34663878 Free PMC article. Review.
References
-
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, et al. Lgr5(+ve) Stem/Progenitor Cells Contribute to Nephron Formation during Kidney Development. Cell Rep. 2012;2:540–552. - PubMed
-
- Barker N, van Es JH, Jaks V, Kasper M, Snippert H, Toftgard R, Clevers H. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:351–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

