Bispidine-amino acid conjugates act as a novel scaffold for the design of antivirals that block Japanese encephalitis virus replication
- PMID: 23350007
- PMCID: PMC3547849
- DOI: 10.1371/journal.pntd.0002005
Bispidine-amino acid conjugates act as a novel scaffold for the design of antivirals that block Japanese encephalitis virus replication
Erratum in
-
Correction: Bispidine-Amino Acid Conjugates Act as a Novel Scaffold for the Design of Antivirals That Block Japanese Encephalitis Virus Replication.PLoS Negl Trop Dis. 2024 Jan 25;18(1):e0011917. doi: 10.1371/journal.pntd.0011917. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38271319 Free PMC article.
Abstract
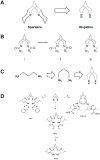
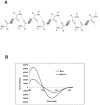
Background: Japanese encephalitis virus (JEV) is a major cause of viral encephalitis in South and South-East Asia. Lack of antivirals and non-availability of affordable vaccines in these endemic areas are a major setback in combating JEV and other closely related viruses such as West Nile virus and dengue virus. Protein secondary structure mimetics are excellent candidates for inhibiting the protein-protein interactions and therefore serve as an attractive tool in drug development. We synthesized derivatives containing the backbone of naturally occurring lupin alkaloid, sparteine, which act as protein secondary structure mimetics and show that these compounds exhibit antiviral properties.
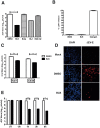
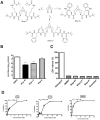
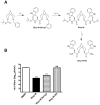
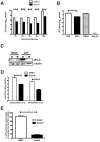
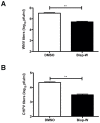
Methodology/principal findings: In this study we have identified 3,7-diazabicyclo[3.3.1]nonane, commonly called bispidine, as a privileged scaffold to synthesize effective antiviral agents. We have synthesized derivatives of bispidine conjugated with amino acids and found that hydrophobic amino acid residues showed antiviral properties against JEV. We identified a tryptophan derivative, Bisp-W, which at 5 µM concentration inhibited JEV infection in neuroblastoma cells by more than 100-fold. Viral inhibition was at a stage post-entry and prior to viral protein translation possibly at viral RNA replication. We show that similar concentration of Bisp-W was capable of inhibiting viral infection of two other encephalitic viruses namely, West Nile virus and Chandipura virus.
Conclusions/significance: We have demonstrated that the amino-acid conjugates of 3,7-diazabicyclo[3.3.1]nonane can serve as a molecular scaffold for development of potent antivirals against encephalitic viruses. Our findings will provide a novel platform to develop effective inhibitors of JEV and perhaps other RNA viruses causing encephalitis.
Conflict of interest statement
Two patent applications are pending related to this study with provisional patent application numbers 2784/Del/2011 and 2786/Del/2011.
Figures
Similar articles
-
N-methylisatin-beta-thiosemicarbazone derivative (SCH 16) is an inhibitor of Japanese encephalitis virus infection in vitro and in vivo.Virol J. 2008 May 22;5:64. doi: 10.1186/1743-422X-5-64. Virol J. 2008. PMID: 18498627 Free PMC article.
-
GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells.J Virol. 2017 Feb 28;91(6):e02274-16. doi: 10.1128/JVI.02274-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28053106 Free PMC article.
-
Screening of FDA-Approved Drugs for Inhibitors of Japanese Encephalitis Virus Infection.J Virol. 2017 Oct 13;91(21):e01055-17. doi: 10.1128/JVI.01055-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814523 Free PMC article.
-
Flaviviruses, an expanding threat in public health: focus on dengue, West Nile, and Japanese encephalitis virus.J Neurovirol. 2014 Dec;20(6):539-60. doi: 10.1007/s13365-014-0285-z. Epub 2014 Oct 7. J Neurovirol. 2014. PMID: 25287260 Free PMC article. Review.
-
Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses.Vaccine. 2010 Jan 8;28(3):632-49. doi: 10.1016/j.vaccine.2009.09.098. Epub 2009 Oct 4. Vaccine. 2010. PMID: 19808029 Review.
Cited by
-
Versatile Bispidine-Based Bifunctional Chelators for 64 CuII -Labelling of Biomolecules.Chemistry. 2020 Feb 11;26(9):1989-2001. doi: 10.1002/chem.201904654. Epub 2020 Jan 9. Chemistry. 2020. PMID: 31755596 Free PMC article.
-
Adaptor protein complexes-1 and 3 are involved at distinct stages of flavivirus life-cycle.Sci Rep. 2013;3:1813. doi: 10.1038/srep01813. Sci Rep. 2013. PMID: 23657274 Free PMC article.
-
Synthesis of non-symmetric N-benzylbispidinol amides and study of their inhibitory activity against the main protease of the SARS-CoV-2 virus.Russ Chem Bull. 2023;72(1):239-247. doi: 10.1007/s11172-023-3729-x. Epub 2023 Feb 14. Russ Chem Bull. 2023. PMID: 36817558 Free PMC article.
-
Neutralizing antibodies from prior exposure to dengue virus negatively correlate with viremia on re-infection.Commun Med (Lond). 2023 Oct 19;3(1):148. doi: 10.1038/s43856-023-00378-7. Commun Med (Lond). 2023. PMID: 37857747 Free PMC article.
-
Amino acid-derived defense metabolites from plants: A potential source to facilitate novel antimicrobial development.J Biol Chem. 2021 Jan-Jun;296:100438. doi: 10.1016/j.jbc.2021.100438. Epub 2021 Feb 19. J Biol Chem. 2021. PMID: 33610552 Free PMC article. Review.
References
-
- Lindenbach BD, Thiel HJ, Rice CM (2007) Flaviviridae: The Viruses and Their Replication. Fields Virology I: 1101–1152.
-
- Medigeshi GR (2011) Mosquito-borne flaviviruses: overview of viral life-cycle and host–virus interactions. Future Virology 6: 1075–1089.
-
- Liskamp RMJ (1994) A New Application of Modified Peptides and Peptidomimetics: Potential Anticancer Agents. Angewandte Chemie International Edition in English 33: 305–307.
-
- Loregian A, Marsden HS, Palu G (2002) Protein-protein interactions as targets for antiviral chemotherapy. Reviews in medical virology 12: 239–262. - PubMed
-
- Tsantrizos YS (2008) Peptidomimetic Therapeutic Agents Targeting the Protease Enzyme of the Human Immunodeficiency Virus and Hepatitis C Virus. Accounts of Chemical Research 41: 1252–1263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

