Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole
- PMID: 23349930
- PMCID: PMC3547880
- DOI: 10.1371/journal.pone.0054566
Effector protein translocation by the Coxiella burnetii Dot/Icm type IV secretion system requires endocytic maturation of the pathogen-occupied vacuole
Abstract
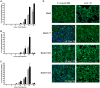
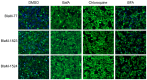
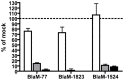
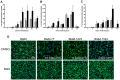
The human pathogen Coxiella burnetii encodes a type IV secretion system called Dot/Icm that is essential for intracellular replication. The Dot/Icm system delivers bacterial effector proteins into the host cytosol during infection. The effector proteins delivered by C. burnetii are predicted to have important functions during infection, but when these proteins are needed during infection has not been clearly defined. Here, we use a reporter system consisting of fusion proteins that have a β-lactamase enzyme (BlaM) fused to C. burnetii effector proteins to study protein translocation by the Dot/Icm system. Translocation of BlaM fused to the effector proteins CBU0077, CBU1823 and CBU1524 was not detected until 8-hours after infection of HeLa cells, which are permissive for C. burnetii replication. Translocation of these effector fusion proteins by the Dot/Icm system required acidification of the Coxiella-containing vacuole. Silencing of the host genes encoding the membrane transport regulators Rab5 or Rab7 interfered with effector translocation, which indicates that effectors are not translocated until bacteria traffic to a late endocytic compartment in the host cell. Similar requirements for effector translocation were discerned in bone marrow macrophages derived from C57BL/6 mice, which are primary cells that restrict the intracellular replication of C. burnetii. In addition to requiring endocytic maturation of the vacuole for Dot/Icm-mediated translocation of effectors, bacterial transcription was required for this process. Thus, translocation of effector proteins by the C. burnetii Dot/Icm system occurs after acidification of the CCV and maturation of this specialized organelle to a late endocytic compartment. This indicates that creation of the specialized vacuole in which C. burnetii replicates represents a two-stage process mediated initially by host factors that regulate endocytic maturation and then by bacterial effectors delivered into host cells after bacteria establish residency in a lysosome-derived organelle.
Conflict of interest statement
Figures
Similar articles
-
Host pathways important for Coxiella burnetii infection revealed by genome-wide RNA interference screening.mBio. 2013 Jan 29;4(1):e00606-12. doi: 10.1128/mBio.00606-12. mBio. 2013. PMID: 23362322 Free PMC article.
-
Coxiella burnetii effector proteins that localize to the parasitophorous vacuole membrane promote intracellular replication.Infect Immun. 2015 Feb;83(2):661-70. doi: 10.1128/IAI.02763-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422265 Free PMC article.
-
A screen of Coxiella burnetii mutants reveals important roles for Dot/Icm effectors and host autophagy in vacuole biogenesis.PLoS Pathog. 2014 Jul 31;10(7):e1004286. doi: 10.1371/journal.ppat.1004286. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 25080348 Free PMC article.
-
Coxiella burnetii secretion systems.Adv Exp Med Biol. 2012;984:171-97. doi: 10.1007/978-94-007-4315-1_9. Adv Exp Med Biol. 2012. PMID: 22711632 Review.
-
Coxiella type IV secretion and cellular microbiology.Curr Opin Microbiol. 2009 Feb;12(1):74-80. doi: 10.1016/j.mib.2008.11.005. Epub 2009 Jan 12. Curr Opin Microbiol. 2009. PMID: 19144560 Free PMC article. Review.
Cited by
-
Interactions between the Coxiella burnetii parasitophorous vacuole and the endoplasmic reticulum involve the host protein ORP1L.Cell Microbiol. 2017 Jan;19(1):10.1111/cmi.12637. doi: 10.1111/cmi.12637. Epub 2016 Jul 15. Cell Microbiol. 2017. PMID: 27345457 Free PMC article.
-
Coxiella burnetii inhibits nuclear translocation of TFEB, the master transcription factor for lysosomal biogenesis.J Bacteriol. 2024 Aug 22;206(8):e0015024. doi: 10.1128/jb.00150-24. Epub 2024 Jul 26. J Bacteriol. 2024. PMID: 39057917 Free PMC article.
-
Illuminating Targets of Bacterial Secretion.PLoS Pathog. 2015 Aug 6;11(8):e1004981. doi: 10.1371/journal.ppat.1004981. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26247771 Free PMC article. Review. No abstract available.
-
Caspase-8 activity mediates TNFα production and restricts Coxiella burnetii replication during murine macrophage infection.bioRxiv [Preprint]. 2024 Feb 3:2024.02.02.578698. doi: 10.1101/2024.02.02.578698. bioRxiv. 2024. Update in: Infect Immun. 2024 Jul 11;92(7):e0005324. doi: 10.1128/iai.00053-24. PMID: 38352389 Free PMC article. Updated. Preprint.
-
Biogenesis of the Spacious Coxiella-Containing Vacuole Depends on Host Transcription Factors TFEB and TFE3.Infect Immun. 2020 Feb 20;88(3):e00534-19. doi: 10.1128/IAI.00534-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31818957 Free PMC article.
References
-
- Backert S, Meyer TF (2006) Type IV secretion systems and their effectors in bacterial pathogenesis. Curr Opin Microbiol 9: 207–217. - PubMed
-
- Hubber A, Roy CR (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26: 261–283. - PubMed
-
- Segal G, Shuman HA (1999) Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii . Mol Microbiol 33: 669–670. - PubMed
-
- Berger KH, Isberg RR (1993) Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila . Mol Microbiol 7: 7–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

