Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier
- PMID: 23345410
- PMCID: PMC3605985
- DOI: 10.1194/jlr.M034710
Cardiac-specific overexpression of perilipin 5 provokes severe cardiac steatosis via the formation of a lipolytic barrier
Abstract
Cardiac triacylglycerol (TG) catabolism critically depends on the TG hydrolytic activity of adipose triglyceride lipase (ATGL). Perilipin 5 (Plin5) is expressed in cardiac muscle (CM) and has been shown to interact with ATGL and its coactivator comparative gene identification-58 (CGI-58). Furthermore, ectopic Plin5 expression increases cellular TG content and Plin5-deficient mice exhibit reduced cardiac TG levels. In this study we show that mice with cardiac muscle-specific overexpression of perilipin 5 (CM-Plin5) massively accumulate TG in CM, which is accompanied by moderately reduced fatty acid (FA) oxidizing gene expression levels. Cardiac lipid droplet (LD) preparations from CM of CM-Plin5 mice showed reduced ATGL- and hormone-sensitive lipase-mediated TG mobilization implying that Plin5 overexpression restricts cardiac lipolysis via the formation of a lipolytic barrier. To test this hypothesis, we analyzed TG hydrolytic activities in preparations of Plin5-, ATGL-, and CGI-58-transfected cells. In vitro ATGL-mediated TG hydrolysis of an artificial micellar TG substrate was not inhibited by the presence of Plin5, whereas Plin5-coated LDs were resistant toward ATGL-mediated TG catabolism. These findings strongly suggest that Plin5 functions as a lipolytic barrier to protect the cardiac TG pool from uncontrolled TG mobilization and the excessive release of free FAs.
Figures
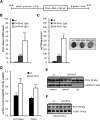
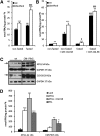
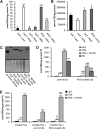
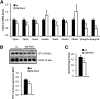

Comment in
-
Perilipin 5: putting the brakes on lipolysis.J Lipid Res. 2013 Apr;54(4):876-7. doi: 10.1194/jlr.E036962. Epub 2013 Feb 17. J Lipid Res. 2013. PMID: 23417737 Free PMC article. No abstract available.
Similar articles
-
The interplay of protein kinase A and perilipin 5 regulates cardiac lipolysis.J Biol Chem. 2015 Jan 16;290(3):1295-306. doi: 10.1074/jbc.M114.604744. Epub 2014 Nov 22. J Biol Chem. 2015. PMID: 25418045 Free PMC article.
-
Functional cardiac lipolysis in mice critically depends on comparative gene identification-58.J Biol Chem. 2013 Apr 5;288(14):9892-9904. doi: 10.1074/jbc.M112.420620. Epub 2013 Feb 14. J Biol Chem. 2013. PMID: 23413028 Free PMC article.
-
Low cardiac lipolysis reduces mitochondrial fission and prevents lipotoxic heart dysfunction in Perilipin 5 mutant mice.Cardiovasc Res. 2020 Feb 1;116(2):339-352. doi: 10.1093/cvr/cvz119. Cardiovasc Res. 2020. PMID: 31166588 Free PMC article.
-
Piecing together the puzzle of perilipin proteins and skeletal muscle lipolysis.Appl Physiol Nutr Metab. 2015 Jul;40(7):641-51. doi: 10.1139/apnm-2014-0485. Epub 2015 Feb 26. Appl Physiol Nutr Metab. 2015. PMID: 25971423 Review.
-
Fate of fat: the role of adipose triglyceride lipase in lipolysis.Biochim Biophys Acta. 2009 Jun;1791(6):494-500. doi: 10.1016/j.bbalip.2008.10.005. Epub 2008 Oct 29. Biochim Biophys Acta. 2009. PMID: 19010445 Review.
Cited by
-
The Unrestrained Overeating Behavior and Clinical Perspective.Adv Exp Med Biol. 2024;1460:167-198. doi: 10.1007/978-3-031-63657-8_6. Adv Exp Med Biol. 2024. PMID: 39287852 Review.
-
Of mice and men: The physiological role of adipose triglyceride lipase (ATGL).Biochim Biophys Acta Mol Cell Biol Lipids. 2019 Jun;1864(6):880-899. doi: 10.1016/j.bbalip.2018.10.008. Epub 2018 Oct 25. Biochim Biophys Acta Mol Cell Biol Lipids. 2019. PMID: 30367950 Free PMC article. Review.
-
Perilipin 5, a lipid droplet protein adapted to mitochondrial energy utilization.Curr Opin Lipidol. 2014 Apr;25(2):110-7. doi: 10.1097/MOL.0000000000000057. Curr Opin Lipidol. 2014. PMID: 24535284 Free PMC article. Review.
-
Comparative gene identification-58/α/β hydrolase domain 5: more than just an adipose triglyceride lipase activator?Curr Opin Lipidol. 2014 Apr;25(2):102-9. doi: 10.1097/MOL.0000000000000058. Curr Opin Lipidol. 2014. PMID: 24565921 Free PMC article. Review.
-
The hepatitis C virus core protein inhibits adipose triglyceride lipase (ATGL)-mediated lipid mobilization and enhances the ATGL interaction with comparative gene identification 58 (CGI-58) and lipid droplets.J Biol Chem. 2014 Dec 26;289(52):35770-80. doi: 10.1074/jbc.M114.587816. Epub 2014 Nov 7. J Biol Chem. 2014. PMID: 25381252 Free PMC article.
References
-
- Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

