Activation of p53 by chemotherapeutic agents enhances reovirus oncolysis
- PMID: 23342061
- PMCID: PMC3546971
- DOI: 10.1371/journal.pone.0054006
Activation of p53 by chemotherapeutic agents enhances reovirus oncolysis
Abstract
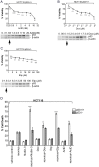
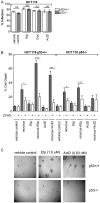
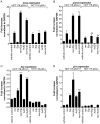
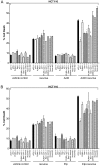
Mammalian reovirus is a benign virus that possesses the natural ability to preferentially infect and kill cancer cells (reovirus oncolysis). Reovirus exploits aberrant Ras signalling in many human cancers to promote its own replication and spread. In vitro and in vivo studies using reovirus either singly or in combination with anti-cancer drugs have shown very encouraging results. Presently, a number of reovirus combination therapies are undergoing clinical trials for a variety of cancers. Previously we showed that accumulation of the tumor suppressor protein p53 by Nutlin-3a (a specific p53 stabilizer) enhanced reovirus-induced apoptosis, and resulted in significantly higher levels of reovirus dissemination. In this study, we examined the role of p53 in combination therapies involving reovirus and chemotherapeutic drugs. We showed that sub-lethal concentrations of traditional chemotherapy drugs actinomycin D or etoposide, but not doxorubicin, enhanced reovirus-induced apoptosis in a p53-dependent manner. Furthermore, NF-κB activation and expression of p53-target genes (p21 and bax) were important for the p53-dependent enhancement of cell death. Our results show that p53 status affects the efficacy of combination therapy involving reovirus. Choosing the right combination partner for reovirus and a low dosage of the drug may help to both enhance reovirus-induced cancer elimination and reduce drug toxicity.
Conflict of interest statement
Figures
Similar articles
-
Stabilisation of p53 enhances reovirus-induced apoptosis and virus spread through p53-dependent NF-κB activation.Br J Cancer. 2011 Sep 27;105(7):1012-22. doi: 10.1038/bjc.2011.325. Epub 2011 Aug 23. Br J Cancer. 2011. PMID: 21863032 Free PMC article.
-
The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status.Oncogene. 2010 Jul 8;29(27):3990-6. doi: 10.1038/onc.2010.137. Epub 2010 May 17. Oncogene. 2010. PMID: 20473328 Free PMC article.
-
Oncolytic reovirus induces intracellular redistribution of Ras to promote apoptosis and progeny virus release.Oncogene. 2016 Feb 11;35(6):771-82. doi: 10.1038/onc.2015.136. Epub 2015 May 11. Oncogene. 2016. PMID: 25961930
-
Oncolytic viral therapy using reovirus.Methods Mol Biol. 2009;542:607-34. doi: 10.1007/978-1-59745-561-9_31. Methods Mol Biol. 2009. PMID: 19565924 Review.
-
Oncolytic Viral Therapy Using Reovirus.Methods Mol Biol. 2015;1317:187-223. doi: 10.1007/978-1-4939-2727-2_12. Methods Mol Biol. 2015. PMID: 26072409 Review.
Cited by
-
Closely related reovirus lab strains induce opposite expression of RIG-I/IFN-dependent versus -independent host genes, via mechanisms of slow replication versus polymorphisms in dsRNA binding σ3 respectively.PLoS Pathog. 2020 Sep 21;16(9):e1008803. doi: 10.1371/journal.ppat.1008803. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32956403 Free PMC article.
-
NF-κB Signaling in Targeting Tumor Cells by Oncolytic Viruses-Therapeutic Perspectives.Cancers (Basel). 2018 Nov 8;10(11):426. doi: 10.3390/cancers10110426. Cancers (Basel). 2018. PMID: 30413032 Free PMC article. Review.
-
Effect of COL4A1 Expression on the Survival of Neoadjuvant Chemotherapy Breast Cancer Patients.J Oncol. 2020 May 14;2020:5209695. doi: 10.1155/2020/5209695. eCollection 2020. J Oncol. 2020. PMID: 32565804 Free PMC article.
-
p38 Mitogen-Activated Protein Kinase Signaling Enhances Reovirus Replication by Facilitating Efficient Virus Entry, Capsid Uncoating, and Postuncoating Steps.J Virol. 2023 Feb 28;97(2):e0000923. doi: 10.1128/jvi.00009-23. Epub 2023 Feb 6. J Virol. 2023. PMID: 36744961 Free PMC article.
-
Oncolytic activity of reovirus in HPV positive and negative head and neck squamous cell carcinoma.J Otolaryngol Head Neck Surg. 2015 Feb 24;44(1):8. doi: 10.1186/s40463-015-0062-x. J Otolaryngol Head Neck Surg. 2015. PMID: 25890191 Free PMC article.
References
-
- Hashiro G, Loh PC, Yau JT (1977) The preferential cytotoxicity of reovirus for certain transformed cell lines. Arch. Virol. 54(4): 307–315. - PubMed
-
- Coffey MC, Strong JE, Forsyth PA, Lee PW (1998) Reovirus therapy of tumors with activated Ras pathway. Science 282(5392): 1332–1334. - PubMed
-
- Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW (2007) Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol. Ther. 15(8): 1522–1530. - PubMed
-
- Shmulevitz M, Pan LZ, Garant K, Pan D, Lee PW (2010) Oncogenic Ras promotes reovirus spread by suppressing IFN-beta production through negative regulation of RIG-I signaling. Cancer Res. 70(12): 4912–4921. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

