ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia
- PMID: 23341542
- PMCID: PMC3606065
- DOI: 10.1182/blood-2013-01-475855
ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia
Abstract
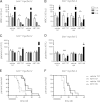
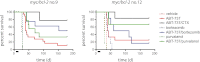
BH3-only proteins trigger the stress apoptosis pathway and chemical mimetics have great potential for cancer therapy. BH3-only proteins inhibit antiapoptotic members of the Bcl-2 family. Promising BH3 mimetic ABT-737 and the related orally available compound ABT-263 (navitoclax) bind avidly to antiapoptotic Bcl-2, Bcl-xL, and Bcl-w. However, their interaction with Bcl-xL provokes thrombocytopenia, which has proven to be the dose-limiting toxicity. We have tested the efficacy of ABT-199, a new Bcl-2-specific BH3 mimetic, against aggressive progenitor cell lymphomas derived from bitransgenic myc/bcl-2 mice. As a single agent, ABT-199 was as effective as ABT-737 in prolonging survival of immunocompetent tumor-bearing mice without causing thrombocytopenia. Both drugs acted rapidly but, contrary to prevailing models, their apoptotic activity did not rely upon the BH3-only protein Bim. When ABT-737 was combined with the proteosome inhibitor bortezomib or CDK inhibitor purvalanol, many treated animals achieved long-term remission.
Figures
Similar articles
-
Impact of elevated anti-apoptotic MCL-1 and BCL-2 on the development and treatment of MLL-AF9 AML in mice.Cell Death Differ. 2019 Jul;26(7):1316-1331. doi: 10.1038/s41418-018-0209-1. Epub 2018 Nov 23. Cell Death Differ. 2019. PMID: 30470795 Free PMC article.
-
In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):17961-6. doi: 10.1073/pnas.0809957105. Epub 2008 Nov 11. Proc Natl Acad Sci U S A. 2008. PMID: 19004807 Free PMC article.
-
Impact of BH3-mimetics on Human and Mouse Blood Leukocytes: A Comparative Study.Sci Rep. 2020 Jan 14;10(1):222. doi: 10.1038/s41598-019-57000-x. Sci Rep. 2020. PMID: 31937836 Free PMC article.
-
Selective Bcl-2 inhibition to treat chronic lymphocytic leukemia and non-Hodgkin lymphoma.Clin Adv Hematol Oncol. 2014 Apr;12(4):224-9. Clin Adv Hematol Oncol. 2014. PMID: 25003352 Review.
-
BH3 mimetics: status of the field and new developments.Mol Cancer Ther. 2013 Sep;12(9):1691-700. doi: 10.1158/1535-7163.MCT-13-0058. Epub 2013 Aug 23. Mol Cancer Ther. 2013. PMID: 23974697 Review.
Cited by
-
Intracellular delivery system for antibody-Peptide drug conjugates.Mol Ther. 2015 May;23(5):907-917. doi: 10.1038/mt.2015.22. Epub 2015 Feb 11. Mol Ther. 2015. PMID: 25669432 Free PMC article.
-
Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells.Cell Death Differ. 2014 Jul;21(7):1170-7. doi: 10.1038/cdd.2014.37. Epub 2014 Mar 28. Cell Death Differ. 2014. PMID: 24682005 Free PMC article.
-
The IGF-1 receptor inhibitor picropodophyllin potentiates the anti-myeloma activity of a BH3-mimetic.Oncotarget. 2014 Nov 30;5(22):11193-208. doi: 10.18632/oncotarget.1933. Oncotarget. 2014. PMID: 25008202 Free PMC article.
-
Critical B-lymphoid cell intrinsic role of endogenous MCL-1 in c-MYC-induced lymphomagenesis.Cell Death Dis. 2016 Mar 10;7(3):e2132. doi: 10.1038/cddis.2016.43. Cell Death Dis. 2016. PMID: 26962682 Free PMC article.
-
Precision therapy for lymphoma--current state and future directions.Nat Rev Clin Oncol. 2014 Oct;11(10):585-96. doi: 10.1038/nrclinonc.2014.137. Epub 2014 Aug 19. Nat Rev Clin Oncol. 2014. PMID: 25135367 Review.
References
-
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. - PubMed
-
- Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–3428. - PubMed
-
- Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

