The cellular prion protein traps Alzheimer's Aβ in an oligomeric form and disassembles amyloid fibers
- PMID: 23335053
- PMCID: PMC3767752
- DOI: 10.1096/fj.12-222588
The cellular prion protein traps Alzheimer's Aβ in an oligomeric form and disassembles amyloid fibers
Abstract
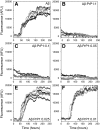
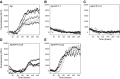
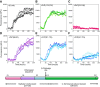
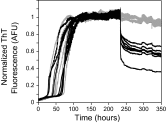
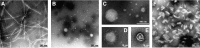
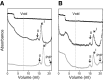
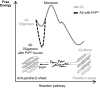
There is now strong evidence to show that the presence of the cellular prion protein (PrP(C)) mediates amyloid-β (Aβ) neurotoxicity in Alzheimer's disease (AD). Here, we probe the molecular details of the interaction between PrP(C) and Aβ and discover that substoichiometric amounts of PrP(C), as little as 1/20, relative to Aβ will strongly inhibit amyloid fibril formation. This effect is specific to the unstructured N-terminal domain of PrP(C). Electron microscopy indicates PrP(C) is able to trap Aβ in an oligomeric form. Unlike fibers, this oligomeric Aβ contains antiparallel β sheet and binds to a oligomer specific conformational antibody. Our NMR studies show that a specific region of PrP(C), notably residues 95-113, binds to Aβ oligomers, but only once Aβ misfolds. The ability of PrP(C) to trap and concentrate Aβ in an oligomeric form and disassemble mature fibers suggests a mechanism by which PrP(C) might confer Aβ toxicity in AD, as oligomers are thought to be the toxic form of Aβ. Identification of a specific recognition site on PrP(C) that traps Aβ in an oligomeric form is potentially a therapeutic target for the treatment of Alzheimer's disease.
Figures



Similar articles
-
Prion protein stabilizes amyloid-β (Aβ) oligomers and enhances Aβ neurotoxicity in a Drosophila model of Alzheimer's disease.J Biol Chem. 2018 Aug 24;293(34):13090-13099. doi: 10.1074/jbc.RA118.003319. Epub 2018 Jun 10. J Biol Chem. 2018. PMID: 29887525 Free PMC article.
-
Cellular prion protein targets amyloid-β fibril ends via its C-terminal domain to prevent elongation.J Biol Chem. 2017 Oct 13;292(41):16858-16871. doi: 10.1074/jbc.M117.789990. Epub 2017 Aug 23. J Biol Chem. 2017. PMID: 28842494 Free PMC article.
-
Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity.J Neurochem. 2016 Apr;137(1):88-100. doi: 10.1111/jnc.13514. Epub 2016 Mar 2. J Neurochem. 2016. PMID: 26710111
-
Physicochemical characteristics of soluble oligomeric Abeta and their pathologic role in Alzheimer's disease.Neurol Res. 2005 Dec;27(8):869-81. doi: 10.1179/016164105X49436. Neurol Res. 2005. PMID: 16354549 Review.
-
Beta-amyloid oligomers and cellular prion protein in Alzheimer's disease.J Mol Med (Berl). 2010 Apr;88(4):331-8. doi: 10.1007/s00109-009-0568-7. Epub 2009 Dec 4. J Mol Med (Berl). 2010. PMID: 19960174 Free PMC article. Review.
Cited by
-
Interaction between prion protein and Aβ amyloid fibrils revisited.ACS Chem Neurosci. 2014 May 21;5(5):340-5. doi: 10.1021/cn500019c. Epub 2014 Apr 1. ACS Chem Neurosci. 2014. PMID: 24669873 Free PMC article.
-
Soluble Aβ aggregates can inhibit prion propagation.Open Biol. 2017 Nov;7(11):170158. doi: 10.1098/rsob.170158. Open Biol. 2017. PMID: 29142106 Free PMC article.
-
Amyloid β oligomers (AβOs) in Alzheimer's disease.J Neural Transm (Vienna). 2018 Feb;125(2):177-191. doi: 10.1007/s00702-017-1820-x. Epub 2017 Dec 1. J Neural Transm (Vienna). 2018. PMID: 29196815 Review.
-
Iron is a specific cofactor for distinct oxidation- and aggregation-dependent Aβ toxicity mechanisms in a Drosophila model.Dis Model Mech. 2015 Jul 1;8(7):657-67. doi: 10.1242/dmm.019042. Epub 2015 Apr 23. Dis Model Mech. 2015. PMID: 26035384 Free PMC article.
-
A hydrogel biosensor for high selective and sensitive detection of amyloid-beta oligomers.Int J Nanomedicine. 2018 Feb 8;13:843-856. doi: 10.2147/IJN.S152163. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29467574 Free PMC article.
References
-
- Hardy J., Selkoe D. J. (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 - PubMed
-
- Walsh D. M., Klyubin I., Fadeeva J. V., Cullen W. K., Anwyl R., Wolfe M. S., Rowan M. J., Selkoe D. J. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 - PubMed
-
- Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 95, 6448–6453 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

