A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors
- PMID: 23334295
- PMCID: PMC3567489
- DOI: 10.1038/emboj.2012.356
A structural basis for kinetochore recruitment of the Ndc80 complex via two distinct centromere receptors
Abstract
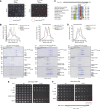
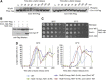
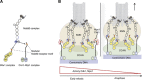
The Ndc80 complex is the key microtubule-binding element of the kinetochore. In contrast to the well-characterized interaction of Ndc80-Nuf2 heads with microtubules, little is known about how the Spc24-25 heterodimer connects to centromeric chromatin. Here, we present molecular details of Spc24-25 in complex with the histone-fold protein Cnn1/CENP-T illustrating how this connection ultimately links microtubules to chromosomes. The conserved Ndc80 receptor motif of Cnn1 is bound as an α helix in a hydrophobic cleft at the interface between Spc24 and Spc25. Point mutations that disrupt the Ndc80-Cnn1 interaction also abrogate binding to the Mtw1 complex and are lethal in yeast. We identify a Cnn1-related motif in the Dsn1 subunit of the Mtw1 complex, necessary for Ndc80 binding and essential for yeast growth. Replacing this region with the Cnn1 peptide restores viability demonstrating functionality of the Ndc80-binding module in different molecular contexts. Finally, phosphorylation of the Cnn1 N-terminus coordinates the binding of the two competing Ndc80 interaction partners. Together, our data provide structural insights into the modular binding mechanism of the Ndc80 complex to its centromere recruiters.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

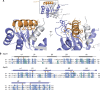



Similar articles
-
The Mps1 kinase modulates the recruitment and activity of Cnn1(CENP-T) at Saccharomyces cerevisiae kinetochores.Genetics. 2015 May;200(1):79-90. doi: 10.1534/genetics.115.175786. Epub 2015 Feb 25. Genetics. 2015. PMID: 25716979 Free PMC article.
-
How the kinetochore couples microtubule force and centromere stretch to move chromosomes.Nat Cell Biol. 2016 Apr;18(4):382-92. doi: 10.1038/ncb3323. Epub 2016 Mar 14. Nat Cell Biol. 2016. PMID: 26974660 Free PMC article.
-
Orientation and structure of the Ndc80 complex on the microtubule lattice.J Cell Biol. 2008 Sep 22;182(6):1055-61. doi: 10.1083/jcb.200804170. Epub 2008 Sep 15. J Cell Biol. 2008. PMID: 18794333 Free PMC article.
-
Molecular architecture and connectivity of the budding yeast Mtw1 kinetochore complex.J Mol Biol. 2011 Jan 14;405(2):548-59. doi: 10.1016/j.jmb.2010.11.012. Epub 2010 Nov 12. J Mol Biol. 2011. PMID: 21075115 Free PMC article.
-
Protein kinases in mitotic phosphorylation of budding yeast CENP-A.Curr Genet. 2019 Dec;65(6):1325-1332. doi: 10.1007/s00294-019-00997-5. Epub 2019 May 22. Curr Genet. 2019. PMID: 31119371 Review.
Cited by
-
Cell cycle control of kinetochore assembly.Nucleus. 2022 Dec;13(1):208-220. doi: 10.1080/19491034.2022.2115246. Nucleus. 2022. PMID: 36037227 Free PMC article. Review.
-
Kinetochore assembly and function through the cell cycle.Chromosoma. 2016 Sep;125(4):645-59. doi: 10.1007/s00412-016-0608-3. Epub 2016 Jul 4. Chromosoma. 2016. PMID: 27376724 Review.
-
NDC80/HEC1 promotes macrophage polarization and predicts glioma prognosis via single-cell RNA-seq and in vitro experiment.CNS Neurosci Ther. 2024 Jul;30(7):e14850. doi: 10.1111/cns.14850. CNS Neurosci Ther. 2024. PMID: 39021287 Free PMC article.
-
An integrated overview of spatiotemporal organization and regulation in mitosis in terms of the proteins in the functional supercomplexes.Front Microbiol. 2014 Oct 29;5:573. doi: 10.3389/fmicb.2014.00573. eCollection 2014. Front Microbiol. 2014. PMID: 25400627 Free PMC article. Review.
-
Structural plasticity of the living kinetochore.J Cell Biol. 2017 Nov 6;216(11):3551-3570. doi: 10.1083/jcb.201703152. Epub 2017 Sep 22. J Cell Biol. 2017. PMID: 28939613 Free PMC article.
References
-
- 4 CCPN. (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50(Pt 5): 760–763 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

