Hypothalamic tanycytes: potential roles in the control of feeding and energy balance
- PMID: 23332797
- PMCID: PMC3605593
- DOI: 10.1016/j.tins.2012.12.008
Hypothalamic tanycytes: potential roles in the control of feeding and energy balance
Abstract
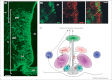
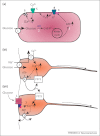
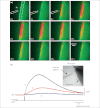
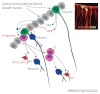
Tanycytes, glial-like cells that line the third ventricle, are emerging as components of the hypothalamic networks that control body weight and energy balance. They contact the cerebrospinal fluid (CSF) and send processes that come into close contact with neurons in the arcuate and ventromedial hypothalamic nuclei. Tanycytes are glucosensitive and are able to respond to transmitters associated with arousal and the drive to feed. At least some tanycytes are stem cells and, in the median eminence, may be stimulated by diet to generate new neurons. The quest is on to understand how tanycytes detect and respond to changes in energy balance and how they communicate with the rest of the nervous system to effect their functions.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Purinergic signaling in hypothalamic tanycytes: potential roles in chemosensing.Semin Cell Dev Biol. 2011 Apr;22(2):237-44. doi: 10.1016/j.semcdb.2011.02.024. Epub 2011 Mar 21. Semin Cell Dev Biol. 2011. PMID: 21396904 Review.
-
ATP-mediated glucosensing by hypothalamic tanycytes.J Physiol. 2011 May 1;589(Pt 9):2275-86. doi: 10.1113/jphysiol.2010.202051. Epub 2011 Mar 8. J Physiol. 2011. PMID: 21486800 Free PMC article.
-
The Versatile Tanycyte: A Hypothalamic Integrator of Reproduction and Energy Metabolism.Endocr Rev. 2018 Jun 1;39(3):333-368. doi: 10.1210/er.2017-00235. Endocr Rev. 2018. PMID: 29351662 Review.
-
A sweet taste receptor-dependent mechanism of glucosensing in hypothalamic tanycytes.Glia. 2017 May;65(5):773-789. doi: 10.1002/glia.23125. Epub 2017 Feb 16. Glia. 2017. PMID: 28205335 Free PMC article.
-
Localization of phospho-tyrosine489-beta-adducin immunoreactivity in the hypothalamic tanycytes and its involvement in energy homeostasis.Brain Res. 2008 Sep 4;1228:97-106. doi: 10.1016/j.brainres.2008.06.093. Epub 2008 Jul 2. Brain Res. 2008. PMID: 18634768
Cited by
-
A review of the peripheral levels of regulation by thyroid hormone.J Comp Physiol B. 2016 Aug;186(6):677-88. doi: 10.1007/s00360-016-0984-2. Epub 2016 Apr 9. J Comp Physiol B. 2016. PMID: 27062031 Review.
-
Planar Organization of Multiciliated Ependymal (E1) Cells in the Brain Ventricular Epithelium.Trends Neurosci. 2016 Aug;39(8):543-551. doi: 10.1016/j.tins.2016.05.004. Epub 2016 Jun 13. Trends Neurosci. 2016. PMID: 27311928 Free PMC article. Review.
-
Tanycytic TSPO inhibition induces lipophagy to regulate lipid metabolism and improve energy balance.Autophagy. 2020 Jul;16(7):1200-1220. doi: 10.1080/15548627.2019.1659616. Epub 2019 Aug 30. Autophagy. 2020. PMID: 31469345 Free PMC article.
-
Developmental and functional relationships between hypothalamic tanycytes and embryonic radial glia.Front Neurosci. 2023 Jan 20;16:1129414. doi: 10.3389/fnins.2022.1129414. eCollection 2022. Front Neurosci. 2023. PMID: 36741057 Free PMC article. Review.
-
Evidence Supporting a Role for the Blood-Cerebrospinal Fluid Barrier Transporting Circulating Ghrelin into the Brain.Mol Neurobiol. 2019 Jun;56(6):4120-4134. doi: 10.1007/s12035-018-1362-8. Epub 2018 Oct 2. Mol Neurobiol. 2019. PMID: 30276663
References
-
- Morton G.J. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. - PubMed
-
- Rodriguez E.M. Hypothalamic tanycytes: a key component of brain–endocrine interaction. Int. Rev. Cytol. 2005;247:89–164. - PubMed
-
- Mathew T.C. Regional analysis of the ependyma of the third ventricle of rat by light and electron microscopy. Anat. Histol. Embryol. 2008;37:9–18. - PubMed
-
- Vigh B. Cerebrospinal fluid-contacting neurons of the central canal and terminal ventricle in various vertebrates. Cell Tissue Res. 1983;231:615–621. - PubMed
-
- Vigh B., Vigh-Teichmann I. Actual problems of the cerebrospinal fluid-contacting neurons. Microsc. Res. Tech. 1998;41:57–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

