A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation
- PMID: 23326584
- PMCID: PMC3542272
- DOI: 10.1371/journal.pone.0054127
A homogeneous, high-throughput assay for phosphatidylinositol 5-phosphate 4-kinase with a novel, rapid substrate preparation
Abstract
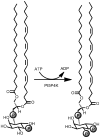
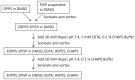
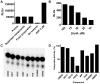
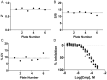
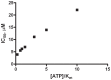
Phosphoinositide kinases regulate diverse cellular functions and are important targets for therapeutic development for diseases, such as diabetes and cancer. Preparation of the lipid substrate is crucial for the development of a robust and miniaturizable lipid kinase assay. Enzymatic assays for phosphoinositide kinases often use lipid substrates prepared from lyophilized lipid preparations by sonication, which result in variability in the liposome size from preparation to preparation. Herein, we report a homogeneous 1536-well luciferase-coupled bioluminescence assay for PI5P4Kα. The substrate preparation is novel and allows the rapid production of a DMSO-containing substrate solution without the need for lengthy liposome preparation protocols, thus enabling the scale-up of this traditionally difficult type of assay. The Z'-factor value was greater than 0.7 for the PI5P4Kα assay, indicating its suitability for high-throughput screening applications. Tyrphostin AG-82 had been identified as an inhibitor of PI5P4Kα by assessing the degree of phospho transfer of γ-(32)P-ATP to PI5P; its inhibitory activity against PI5P4Kα was confirmed in the present miniaturized assay. From a pilot screen of a library of bioactive compounds, another tyrphostin, I-OMe tyrphostin AG-538 (I-OMe-AG-538), was identified as an ATP-competitive inhibitor of PI5P4Kα with an IC(50) of 1 µM, affirming the suitability of the assay for inhibitor discovery campaigns. This homogeneous assay may apply to other lipid kinases and should help in the identification of leads for this class of enzymes by enabling high-throughput screening efforts.
Conflict of interest statement
Figures
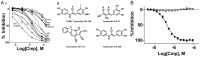
Similar articles
-
Method for Assaying the Lipid Kinase Phosphatidylinositol-5-phosphate 4-kinase α in Quantitative High-Throughput Screening (qHTS) Bioluminescent Format.Methods Mol Biol. 2016;1376:1-9. doi: 10.1007/978-1-4939-3170-5_1. Methods Mol Biol. 2016. PMID: 26552670 Free PMC article.
-
Phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ), a lipid signalling enigma.Adv Biol Regul. 2016 May;61:47-50. doi: 10.1016/j.jbior.2015.11.007. Epub 2015 Dec 2. Adv Biol Regul. 2016. PMID: 26710750 Review.
-
Bioluminescence Methods for Assaying Kinases in Quantitative High-Throughput Screening (qHTS) Format Applied to Yes1 Tyrosine Kinase, Glucokinase, and PI5P4Kα Lipid Kinase.Methods Mol Biol. 2016;1360:47-58. doi: 10.1007/978-1-4939-3073-9_4. Methods Mol Biol. 2016. PMID: 26501901 Free PMC article.
-
High-throughput, cell-free, liposome-based approach for assessing in vitro activity of lipid kinases.J Biomol Screen. 2009 Aug;14(7):838-44. doi: 10.1177/1087057109339205. Epub 2009 Jul 29. J Biomol Screen. 2009. PMID: 19641220
-
Development and application of PI3K assays for novel drug discovery.Expert Opin Drug Discov. 2015 Feb;10(2):171-86. doi: 10.1517/17460441.2015.997205. Epub 2014 Dec 30. Expert Opin Drug Discov. 2015. PMID: 25547459 Review.
Cited by
-
Structure-Activity Relationship Study of Covalent Pan-phosphatidylinositol 5-Phosphate 4-Kinase Inhibitors.ACS Med Chem Lett. 2019 Nov 3;11(3):346-352. doi: 10.1021/acsmedchemlett.9b00402. eCollection 2020 Mar 12. ACS Med Chem Lett. 2019. PMID: 32184968 Free PMC article.
-
Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality.Nat Commun. 2017 Dec 19;8(1):2200. doi: 10.1038/s41467-017-02287-5. Nat Commun. 2017. PMID: 29259156 Free PMC article.
-
Identification of Novel Plasmodium falciparum Hexokinase Inhibitors with Antiparasitic Activity.Antimicrob Agents Chemother. 2016 Sep 23;60(10):6023-33. doi: 10.1128/AAC.00914-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27458230 Free PMC article.
-
Expanding role of PI5P4Ks in cancer: A promising druggable target.FEBS Lett. 2022 Jan;596(1):3-16. doi: 10.1002/1873-3468.14237. Epub 2021 Dec 7. FEBS Lett. 2022. PMID: 34822164 Free PMC article. Review.
-
Screening assay for small-molecule inhibitors of synaptojanin 1, a synaptic phosphoinositide phosphatase.J Biomol Screen. 2014 Apr;19(4):585-94. doi: 10.1177/1087057113510177. Epub 2013 Nov 1. J Biomol Screen. 2014. PMID: 24186361 Free PMC article.
References
-
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296: 1655–1657. - PubMed
-
- Bunney TD, Katan M (2010) Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nature Reviews Cancer 10: 342–352. - PubMed
-
- Sasaki AT, Firtel RA (2006) Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR. European Journal of Cell Biology 85: 873–895. - PubMed
-
- Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nature Reviews Molecular Cell Biology 13: 195–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

