Short-course antiretroviral therapy in primary HIV infection
- PMID: 23323897
- PMCID: PMC4131004
- DOI: 10.1056/NEJMoa1110039
Short-course antiretroviral therapy in primary HIV infection
Abstract
Background: Short-course antiretroviral therapy (ART) in primary human immunodeficiency virus (HIV) infection may delay disease progression but has not been adequately evaluated.
Methods: We randomly assigned adults with primary HIV infection to ART for 48 weeks, ART for 12 weeks, or no ART (standard of care), with treatment initiated within 6 months after seroconversion. The primary end point was a CD4+ count of less than 350 cells per cubic millimeter or long-term ART initiation.
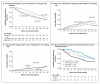
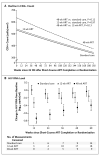
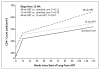
Results: A total of 366 participants (60% men) underwent randomization to 48-week ART (123 participants), 12-week ART (120), or standard care (123), with an average follow-up of 4.2 years. The primary end point was reached in 50% of the 48-week ART group, as compared with 61% in each of the 12-week ART and standard-care groups. The average hazard ratio was 0.63 (95% confidence interval [CI], 0.45 to 0.90; P=0.01) for 48-week ART as compared with standard care and was 0.93 (95% CI, 0.67 to 1.29; P=0.67) for 12-week ART as compared with standard care. The proportion of participants who had a CD4+ count of less than 350 cells per cubic millimeter was 28% in the 48-week ART group, 40% in the 12-week group, and 40% in the standard-care group. Corresponding values for long-term ART initiation were 22%, 21%, and 22%. The median time to the primary end point was 65 weeks (95% CI, 17 to 114) longer with 48-week ART than with standard care. Post hoc analysis identified a trend toward a greater interval between ART initiation and the primary end point the closer that ART was initiated to estimated seroconversion (P=0.09), and 48-week ART conferred a reduction in the HIV RNA level of 0.44 log(10) copies per milliliter (95% CI, 0.25 to 0.64) 36 weeks after the completion of short-course therapy. There were no significant between-group differences in the incidence of the acquired immunodeficiency syndrome, death, or serious adverse events.
Conclusions: A 48-week course of ART in patients with primary HIV infection delayed disease progression, although not significantly longer than the duration of the treatment. There was no evidence of adverse effects of ART interruption on the clinical outcome. (Funded by the Wellcome Trust; SPARTAC Controlled-Trials.com number, ISRCTN76742797, and EudraCT number, 2004-000446-20.).
Figures
Comment in
-
Antiretroviral therapy in early HIV infection.N Engl J Med. 2013 Jan 17;368(3):279-81. doi: 10.1056/NEJMe1213734. N Engl J Med. 2013. PMID: 23323905 No abstract available.
-
Short-course antiretroviral therapy in primary HIV infection.N Engl J Med. 2013 May 23;368(21):2036-7. doi: 10.1056/NEJMc1303486. N Engl J Med. 2013. PMID: 23697518 No abstract available.
-
Short-course antiretroviral therapy in primary HIV infection.N Engl J Med. 2013 May 23;368(21):2036. doi: 10.1056/NEJMc1303486. N Engl J Med. 2013. PMID: 23697519 No abstract available.
-
Antiretroviral therapy in primary HIV infection.J Comp Eff Res. 2013 May;2(3):227-9. doi: 10.2217/cer.13.22. J Comp Eff Res. 2013. PMID: 24236621
Similar articles
-
CD4+ count-guided interruption of antiretroviral treatment.N Engl J Med. 2006 Nov 30;355(22):2283-96. doi: 10.1056/NEJMoa062360. N Engl J Med. 2006. PMID: 17135583 Clinical Trial.
-
Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy.N Engl J Med. 2013 Jan 17;368(3):218-30. doi: 10.1056/NEJMoa1110187. N Engl J Med. 2013. PMID: 23323898 Free PMC article.
-
Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection.N Engl J Med. 2015 Aug 27;373(9):795-807. doi: 10.1056/NEJMoa1506816. Epub 2015 Jul 20. N Engl J Med. 2015. PMID: 26192873 Free PMC article. Clinical Trial.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article. Review.
-
Structured treatment interruptions (STI) in chronic unsuppressed HIV infection in adults.Cochrane Database Syst Rev. 2006 Jul 19;2006(3):CD006148. doi: 10.1002/14651858.CD006148. Cochrane Database Syst Rev. 2006. PMID: 16856117 Free PMC article. Review.
Cited by
-
Lessons learned from U.S. rapid antiretroviral therapy initiation programs.Int J STD AIDS. 2023 Nov;34(13):945-955. doi: 10.1177/09564624231185622. Epub 2023 Jul 17. Int J STD AIDS. 2023. PMID: 37461333 Free PMC article.
-
Human MAIT cells respond to and suppress HIV-1.Elife. 2021 Dec 24;10:e50324. doi: 10.7554/eLife.50324. Elife. 2021. PMID: 34951583 Free PMC article.
-
Duration of HIV-1 viral suppression on cessation of antiretroviral therapy in primary infection correlates with time on therapy.PLoS One. 2013 Oct 25;8(10):e78287. doi: 10.1371/journal.pone.0078287. eCollection 2013. PLoS One. 2013. PMID: 24205183 Free PMC article. Clinical Trial.
-
Second European Round Table on the Future Management of HIV: 10-11 October 2014, Barcelona, Spain.J Virus Erad. 2015 Jul 1;1(3):211-20. doi: 10.1016/S2055-6640(20)30497-0. J Virus Erad. 2015. PMID: 27482415 Free PMC article.
-
Dynamics of immunoglobulin sequence diversity in HIV-1 infected individuals.Philos Trans R Soc Lond B Biol Sci. 2015 Sep 5;370(1676):20140241. doi: 10.1098/rstb.2014.0241. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26194755 Free PMC article.
References
-
- Detels R, Muñoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. JAMA. 1998;280:1497–503. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. - PubMed
-
- Fidler S, Fox J, Porter K, Weber J. Primary HIV infection: to treat or not to treat? Curr Opin Infect Dis. 2008;21:4–10. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
