Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms
- PMID: 23320102
- PMCID: PMC3540071
- DOI: 10.1371/journal.pone.0053766
Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms
Abstract
Background: Aberrant epigenetic patterns are central in the pathogenesis of haematopoietic diseases such as myelodysplastic syndromes (MDS) and acute myeloid leukaemia (AML). Vorinostat is a HDACi which has produced responses in these disorders. The purpose of this study was to address the functional effects of vorinostat in leukemic cell lines and primary AML and MDS myeloid cells and to dissect the genetic and molecular mechanisms by which it exerts its action.
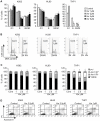
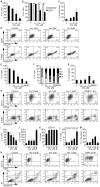
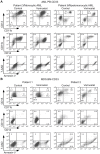
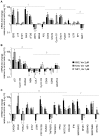
Methodology/principal findings: Functional assays showed vorinostat promoted cell cycle arrest, inhibited growth, and induced apoptosis and differentiation of K562, HL60 and THP-1 and of CD33(+) cells from AML and MDS patients. To explore the genetic mechanism for these effects, we quantified gene expression modulation by vorinostat in these cells. Vorinostat increased expression of genes down-regulated in MDS and/or AML (cFOS, COX2, IER3, p15, RAI3) and suppressed expression of genes over-expressed in these malignancies (AXL, c-MYC, Cyclin D1) and modulated cell cycle and apoptosis genes in a manner which would favor cell cycle arrest, differentiation, and apoptosis of neoplastic cells, consistent with the functional assays. Reporter assays showed transcriptional effect of vorinostat on some of these genes was mediated by proximal promoter elements in GC-rich regions. Vorinostat-modulated expression of some genes was potentiated by mithramycin A, a compound that interferes with SP1 binding to GC-rich DNA sequences, and siRNA-mediated SP1 reduction. ChIP assays revealed vorinostat inhibited DNA binding of SP1 to the proximal promoter regions of these genes. These results suggest vorinostat transcriptional action in some genes is regulated by proximal promoter GC-rich DNA sequences and by SP1.
Conclusion: This study sheds light on the effects of vorinostat in AML and MDS and supports the implementation of clinical trials to explore the use of vorinostat in the treatment of these diseases.
Conflict of interest statement
Figures



Similar articles
-
Arsenic disulfide-triggered apoptosis and erythroid differentiation in myelodysplastic syndrome and acute myeloid leukemia cell lines.Hematology. 2014 Sep;19(6):352-60. doi: 10.1179/1607845413Y.0000000138. Epub 2013 Nov 25. Hematology. 2014. PMID: 24192507
-
Vorinostat induces reactive oxygen species and DNA damage in acute myeloid leukemia cells.PLoS One. 2011;6(6):e20987. doi: 10.1371/journal.pone.0020987. Epub 2011 Jun 10. PLoS One. 2011. PMID: 21695163 Free PMC article.
-
Mechanisms and potential molecular markers of early response to combination epigenetic therapy in patients with myeloid malignancies.Int J Oncol. 2014 Oct;45(4):1742-8. doi: 10.3892/ijo.2014.2555. Epub 2014 Jul 22. Int J Oncol. 2014. PMID: 25051119
-
Vorinostat in acute myeloid leukemia and myelodysplastic syndromes.Expert Opin Investig Drugs. 2011 Feb;20(2):287-95. doi: 10.1517/13543784.2011.542750. Epub 2010 Dec 31. Expert Opin Investig Drugs. 2011. PMID: 21192773 Review.
-
The role of epigenetics in the regulation of apoptosis in myelodysplastic syndromes and acute myeloid leukemia.Crit Rev Oncol Hematol. 2014 Apr;90(1):1-16. doi: 10.1016/j.critrevonc.2013.10.003. Epub 2013 Oct 12. Crit Rev Oncol Hematol. 2014. PMID: 24183913 Review.
Cited by
-
A clinical trial for patients with acute myeloid leukemia or myelodysplastic syndromes not eligible for standard clinical trials.Leukemia. 2017 Feb;31(2):318-324. doi: 10.1038/leu.2016.303. Epub 2016 Oct 31. Leukemia. 2017. PMID: 27795561 Clinical Trial.
-
The targeted histone deacetylase inhibitor tefinostat (CHR-2845) shows selective in vitro efficacy in monocytoid-lineage leukaemias.Oncotarget. 2016 Mar 29;7(13):16650-62. doi: 10.18632/oncotarget.7692. Oncotarget. 2016. PMID: 26934551 Free PMC article.
-
Selective Inhibitors of Histone Deacetylases 1 and 2 Synergize with Azacitidine in Acute Myeloid Leukemia.PLoS One. 2017 Jan 6;12(1):e0169128. doi: 10.1371/journal.pone.0169128. eCollection 2017. PLoS One. 2017. PMID: 28060870 Free PMC article.
-
Naturally occurring benzoic acid derivatives retard cancer cell growth by inhibiting histone deacetylases (HDAC).Cancer Biol Ther. 2017 Jul 3;18(7):492-504. doi: 10.1080/15384047.2017.1324374. Epub 2017 May 16. Cancer Biol Ther. 2017. PMID: 28506198 Free PMC article.
-
Relapsed and refractory pediatric acute myeloid leukemia: current and emerging treatments.Paediatr Drugs. 2014 Apr;16(2):151-68. doi: 10.1007/s40272-013-0048-y. Paediatr Drugs. 2014. PMID: 24158739 Review.
References
-
- Tsiftsoglou AS, Bonovolias ID, Tsiftsoglou SA (2009) Multilevel targeting of hematopoietic stem cell self-renewal, differentiation and apoptosis for leukemia therapy. Pharmacol Ther 122: 264–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

