Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury
- PMID: 23318173
- PMCID: PMC3732190
- DOI: 10.1016/j.bbrc.2013.01.015
Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury
Abstract
Background: Cardiac progenitors (CPC) mediate cardioprotection via paracrine effects. To date, most of studies focused on secreted paracrine proteins. Here we investigated the CPC-derived-exosomes on protecting myocardium from acute ischemia/reperfusion (MI/R) injury.
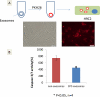
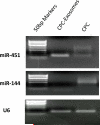
Methods and results: CPC were isolated from mouse heart using two-step protocol. Exosomes were purified from conditional medium, and confirmed by electron micrograph and Western blot using CD63 as a marker. qRT-PCR shows that CPC-exosomes have high level expression of GATA4-responsive-miR-451. Exosomes were ex vivo labeled with PKH26, We observed exosomes can be uptaken by H9C2 cardiomyoblasts with high efficiency after 12 h incubation. CPC-exosomes protect H9C2 from oxidative stress by inhibiting caspase 3/7 activation invitro. In vivo delivery of CPC-exosomes in an acute mouse myocardial ischemia/reperfusion model inhibited cardiomyocyte apoptosis by about 53% in comparison with PBS control (p<0.05).
Conclusion: Our results suggest, for the first time, the CPC-exosomes can be used as a therapeutic vehicle for cardioprotection, and highlights a new perspective for using non-cell exosomes for cardiac disease.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4.Cell Death Dis. 2016 Jun 23;7(6):e2277. doi: 10.1038/cddis.2016.181. Cell Death Dis. 2016. PMID: 27336721 Free PMC article.
-
Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A.Cardiovasc Res. 2018 Jun 1;114(7):992-1005. doi: 10.1093/cvr/cvy055. Cardiovasc Res. 2018. PMID: 29518183
-
Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium.Int J Cardiol. 2015 Aug 1;192:61-9. doi: 10.1016/j.ijcard.2015.05.020. Epub 2015 May 8. Int J Cardiol. 2015. PMID: 26000464 Free PMC article.
-
Exosomes as a Cell-free Therapy for Myocardial Injury Following Acute Myocardial Infarction or Ischemic Reperfusion.Aging Dis. 2022 Dec 1;13(6):1770-1786. doi: 10.14336/AD.2022.0416. eCollection 2022 Dec 1. Aging Dis. 2022. PMID: 36465167 Free PMC article. Review.
-
Research progress of exosomes from different sources in myocardial ischemia.Front Cardiovasc Med. 2024 Sep 12;11:1436764. doi: 10.3389/fcvm.2024.1436764. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39350967 Free PMC article. Review.
Cited by
-
New Insights into the Role of Exosomes in the Heart After Myocardial Infarction.J Cardiovasc Transl Res. 2019 Feb;12(1):18-27. doi: 10.1007/s12265-018-9831-z. Epub 2018 Sep 2. J Cardiovasc Transl Res. 2019. PMID: 30173401 Review.
-
Evaluation and manipulation of tissue and cellular distribution of cardiac progenitor cell-derived extracellular vesicles.Front Pharmacol. 2022 Nov 24;13:1052091. doi: 10.3389/fphar.2022.1052091. eCollection 2022. Front Pharmacol. 2022. PMID: 36506565 Free PMC article.
-
Noncoding RNAs regulating cardiac muscle mass.J Appl Physiol (1985). 2019 Aug 1;127(2):633-644. doi: 10.1152/japplphysiol.00904.2018. Epub 2018 Dec 20. J Appl Physiol (1985). 2019. PMID: 30571279 Free PMC article. Review.
-
Exploring New Kingdoms: The Role of Extracellular Vesicles in Oxi-Inflamm-Aging Related to Cardiorenal Syndrome.Antioxidants (Basel). 2021 Dec 29;11(1):78. doi: 10.3390/antiox11010078. Antioxidants (Basel). 2021. PMID: 35052582 Free PMC article. Review.
-
The Small GTPases Rab27b Regulates Mitochondrial Fatty Acid Oxidative Metabolism of Cardiac Mesenchymal Stem Cells.Front Cell Dev Biol. 2020 Apr 15;8:209. doi: 10.3389/fcell.2020.00209. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32351955 Free PMC article.
References
-
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12313–12318. - PMC - PubMed
-
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circulation research. 2004;95(9):911–921. - PubMed
-
- Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(25):8966–8971. - PMC - PubMed
-
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circulation research. 2009;104(10):1209–1216. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

