Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling
- PMID: 23315163
- PMCID: PMC3596968
- DOI: 10.1182/blood-2012-08-449983
Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling
Abstract
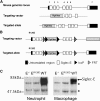
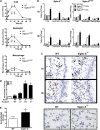
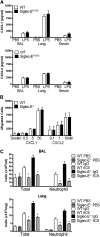
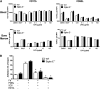
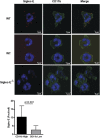
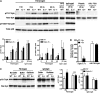
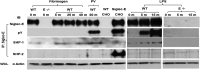
Neutrophil entry into the lung tissues is a key step in host defense to bacterial and yeast infections, but if uncontrolled can lead to severe tissue damage. Here, we demonstrate for the first time that sialic acid binding Ig-like lectin E (siglec-E) functions to selectively regulate early neutrophil recruitment into the lung. In a model of acute lung inflammation induced by aerosolized lipopolysaccharide, siglec-E-deficient mice exhibited exaggerated neutrophil recruitment that was reversed by blockade of the β2 integrin, CD11b. Siglec-E suppressed CD11b "outside-in" signaling, because siglec-E-deficient neutrophils plated on the CD11b ligand fibrinogen showed exaggerated phosphorylation of Syk and p38 mitogen-activated protein kinase. Sialidase treatment of fibrinogen reversed the suppressive effect of siglec-E on CD11b signaling, suggesting that sialic acid recognition by siglec-E is required for its inhibitory function. Siglec-E in neutrophils was constitutively associated with the tyrosine phosphatase SHP-1 and may therefore function to constitutively dampen inflammatory responses of neutrophils. These data reveal that siglec-E is an important negative regulator of neutrophil recruitment to the lung and β2 integrin-dependent signaling. Our findings have implications for the human functional ortholog, siglec-9, and its potential role in regulating inflammatory lung disease.
Figures
Similar articles
-
Siglec-E promotes β2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung.J Biol Chem. 2014 Jul 18;289(29):20370-6. doi: 10.1074/jbc.M114.574624. Epub 2014 Jun 3. J Biol Chem. 2014. PMID: 24895121 Free PMC article.
-
Neutrophils Promote Mycobacterial Trehalose Dimycolate-Induced Lung Inflammation via the Mincle Pathway.PLoS Pathog. 2012;8(4):e1002614. doi: 10.1371/journal.ppat.1002614. Epub 2012 Apr 5. PLoS Pathog. 2012. PMID: 22496642 Free PMC article.
-
Siglec-8 Signals Through a Non-Canonical Pathway to Cause Human Eosinophil Death In Vitro.Front Immunol. 2021 Oct 11;12:737988. doi: 10.3389/fimmu.2021.737988. eCollection 2021. Front Immunol. 2021. PMID: 34721399 Free PMC article.
-
β2 Integrin Signaling Cascade in Neutrophils: More Than a Single Function.Front Immunol. 2021 Feb 18;11:619925. doi: 10.3389/fimmu.2020.619925. eCollection 2020. Front Immunol. 2021. PMID: 33679708 Free PMC article. Review.
-
β2 Integrin Regulation of Neutrophil Functional Plasticity and Fate in the Resolution of Inflammation.Front Immunol. 2021 Mar 30;12:660760. doi: 10.3389/fimmu.2021.660760. eCollection 2021. Front Immunol. 2021. PMID: 33859651 Free PMC article. Review.
Cited by
-
Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies.Proc Natl Acad Sci U S A. 2014 Apr 22;111(16):5998-6003. doi: 10.1073/pnas.1209067111. Epub 2014 Apr 7. Proc Natl Acad Sci U S A. 2014. PMID: 24711415 Free PMC article.
-
Role of negative regulation of immune signaling pathways in neutrophil function.J Leukoc Biol. 2017 Dec 19:10.1002/JLB.3MIR0917-374R. doi: 10.1002/JLB.3MIR0917-374R. Online ahead of print. J Leukoc Biol. 2017. PMID: 29345376 Free PMC article. Review.
-
Lectin galactoside-binding soluble 3 binding protein (LGALS3BP) is a tumor-associated immunomodulatory ligand for CD33-related Siglecs.J Biol Chem. 2014 Nov 28;289(48):33481-91. doi: 10.1074/jbc.M114.593129. Epub 2014 Oct 15. J Biol Chem. 2014. PMID: 25320078 Free PMC article.
-
Regulation of airway inflammation by Siglec-8 and Siglec-9 sialoglycan ligand expression.Curr Opin Allergy Clin Immunol. 2016 Feb;16(1):24-30. doi: 10.1097/ACI.0000000000000234. Curr Opin Allergy Clin Immunol. 2016. PMID: 26694037 Free PMC article. Review.
-
Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating β2-integrin-dependent function in human eosinophils.J Allergy Clin Immunol. 2018 Jun;141(6):2196-2207. doi: 10.1016/j.jaci.2017.08.013. Epub 2017 Sep 6. J Allergy Clin Immunol. 2018. PMID: 28888781 Free PMC article.
References
-
- Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83(2):309–336. - PubMed
-
- Wiggs BR, English D, Quinlan WM, et al. Contributions of capillary pathway size and neutrophil deformability to neutrophil transit through rabbit lungs. J Appl Physiol. 1994;77(1):463–470. - PubMed
-
- Drost EM, Kassabian G, Meiselman HJ, et al. Increased rigidity and priming of polymorphonuclear leukocytes in sepsis. Am J Respir Crit Care Med. 1999;159(6):1696–1702. - PubMed
-
- Moreland JG, Fuhrman RM, Pruessner JA, et al. CD11b and intercellular adhesion molecule-1 are involved in pulmonary neutrophil recruitment in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2002;27(4):474–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

