The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells
- PMID: 23313730
- PMCID: PMC3602316
- DOI: 10.1016/j.neuro.2013.01.002
The effect of manganese on dopamine toxicity and dopamine transporter (DAT) in control and DAT transfected HEK cells
Abstract
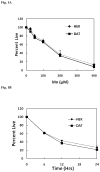
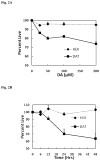
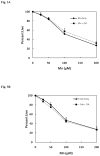
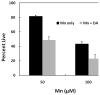
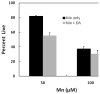
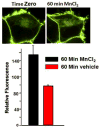
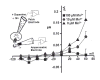
Chronic exposure to Mn results in the development of a neurological disorder known as manganism characterized by neurological deficits resembling that seen in Parkinsonism. Although dopaminergic neurons within the nigrostriatal pathway appear intact, Mn-induced irregularities in DA transmission have been observed including decreased amphetamine-induced DA release and loss of the dopamine transporter (DAT). Results of studies to evaluate the effect of Mn and DA on cell viability in control and DAT-transfected HEK cells reveal that Mn is equally toxic to both cell lines whereas DA was only toxic to cells containing DAT. DA toxicity was saturable suggesting that transport may be rate limiting. When Mn and DA were added simultaneously to the media, cell toxicity was similar to that produced by Mn alone suggesting that Mn may suppress DA uptake in the DAT containing cells. Preincubation of DA prior to the addition of Mn resulted in cell death which was essentially additive with that produced independently by the two agents. Mn was also shown to decrease DA uptake and amphetamine-induced DA efflux in DAT containing cells. Time-lapsed confocal microscopy indicates that Mn can promote trafficking of cell surface DAT into intracellular compartments which may account for the decrease in DA uptake and DA efflux in these cells. Mn-induced internalization of DAT may provide an explanation for disruption in DA transmission previously reported in the striatum.
Copyright © 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
There is no conflict of interest which effects objectivity in regard to publishing this paper.
Figures
Similar articles
-
Down-regulation of LRRK2 in control and DAT transfected HEK cells increases manganese-induced oxidative stress and cell toxicity.Neurotoxicology. 2013 Jul;37:100-7. doi: 10.1016/j.neuro.2013.04.008. Epub 2013 Apr 27. Neurotoxicology. 2013. PMID: 23628791 Free PMC article.
-
Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux.Mol Pharmacol. 2008 Oct;74(4):1101-8. doi: 10.1124/mol.108.048447. Epub 2008 Jul 10. Mol Pharmacol. 2008. PMID: 18617632 Free PMC article.
-
PKC phosphorylates residues in the N-terminal of the DA transporter to regulate amphetamine-induced DA efflux.Neurosci Lett. 2016 May 27;622:78-82. doi: 10.1016/j.neulet.2016.04.051. Epub 2016 Apr 22. Neurosci Lett. 2016. PMID: 27113203 Free PMC article.
-
Phosphorylation of the Amino Terminus of the Dopamine Transporter: Regulatory Mechanisms and Implications for Amphetamine Action.Adv Pharmacol. 2018;82:205-234. doi: 10.1016/bs.apha.2017.09.002. Epub 2017 Oct 25. Adv Pharmacol. 2018. PMID: 29413521 Free PMC article. Review.
-
Dopamine release mediated by the dopamine transporter, facts and consequences.J Neurochem. 2011 Aug;118(4):475-89. doi: 10.1111/j.1471-4159.2011.07335.x. Epub 2011 Jul 1. J Neurochem. 2011. PMID: 21644994 Review.
Cited by
-
Sex Differences in Dopaminergic Vulnerability to Environmental Toxicants - Implications for Parkinson's Disease.Curr Environ Health Rep. 2022 Dec;9(4):563-573. doi: 10.1007/s40572-022-00380-6. Epub 2022 Oct 6. Curr Environ Health Rep. 2022. PMID: 36201109 Free PMC article. Review.
-
Mechanism of Manganese Dysregulation of Dopamine Neuronal Activity.J Neurosci. 2020 Jul 22;40(30):5871-5891. doi: 10.1523/JNEUROSCI.2830-19.2020. Epub 2020 Jun 23. J Neurosci. 2020. PMID: 32576620 Free PMC article.
-
Yeast as a Tool for Deeper Understanding of Human Manganese-Related Diseases.Genes (Basel). 2019 Jul 17;10(7):545. doi: 10.3390/genes10070545. Genes (Basel). 2019. PMID: 31319631 Free PMC article. Review.
-
Consequences of Disturbing Manganese Homeostasis.Int J Mol Sci. 2023 Oct 6;24(19):14959. doi: 10.3390/ijms241914959. Int J Mol Sci. 2023. PMID: 37834407 Free PMC article. Review.
-
Manganese in autism spectrum disorder and attention deficit hyperactivity disorder: The state of the art.Curr Res Toxicol. 2024 Apr 25;6:100170. doi: 10.1016/j.crtox.2024.100170. eCollection 2024. Curr Res Toxicol. 2024. PMID: 38737010 Free PMC article. Review.
References
-
- Chen MK, Lee JS, McGlothan JL, Furukawa E, Adams RJ, Alexander M, Wong DF, Guilarte TR. Acute manganese administration alters dopamine transporter levels in the non-human primate striatum. Neurotoxicology. 2006;27:229–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

