Regulation of NF-κB by ubiquitination
- PMID: 23312890
- PMCID: PMC3594545
- DOI: 10.1016/j.coi.2012.12.005
Regulation of NF-κB by ubiquitination
Abstract
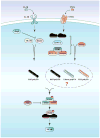
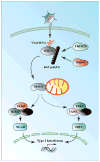
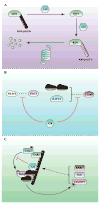
The nuclear factor κ enhancer binding protein (NF-κB) family of transcription factors regulates the expression of a large array of genes involved in diverse cellular processes including inflammation, immunity and cell survival. Activation of NF-κB requires ubiquitination, a highly conserved and versatile modification that can regulate cell signaling through both proteasome dependent and independent mechanisms. Studies in the past few years have provided new insights into the mechanisms underlying regulation of NF-κB by ubiquitination, including the involvement of multiple linkages of ubiquitin, the essential role of ubiquitin binding, and the function of unanchored polyubiquitin chains. In this review, we will focus on recent advances in understanding the role of ubiquitination in NF-κB regulation in various pathways.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The Role of Ubiquitination in NF-κB Signaling during Virus Infection.Viruses. 2021 Jan 20;13(2):145. doi: 10.3390/v13020145. Viruses. 2021. PMID: 33498196 Free PMC article. Review.
-
The role of ubiquitin in NF-kappaB regulatory pathways.Annu Rev Biochem. 2009;78:769-96. doi: 10.1146/annurev.biochem.78.070907.102750. Annu Rev Biochem. 2009. PMID: 19489733 Review.
-
Expanding role of ubiquitination in NF-κB signaling.Cell Res. 2011 Jan;21(1):6-21. doi: 10.1038/cr.2010.170. Epub 2010 Dec 7. Cell Res. 2011. PMID: 21135871 Free PMC article. Review.
-
Linear ubiquitination-mediated NF-κB regulation and its related disorders.J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
-
Chinese Herbal Formula, Huayu Tongbi Fang, Attenuates Inflammatory Proliferation of Rat Synoviocytes Induced by IL-1β by Regulating Proliferation and Differentiation of T Lymphocytes.Evid Based Complement Alternat Med. 2020 May 19;2020:1706837. doi: 10.1155/2020/1706837. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32565847 Free PMC article.
-
The dependency of autophagy and ubiquitin proteasome system during skeletal muscle atrophy.Biophys Rev. 2021 Mar 4;13(2):203-219. doi: 10.1007/s12551-021-00789-7. eCollection 2021 Apr. Biophys Rev. 2021. PMID: 33927785 Free PMC article. Review.
-
Oncogenic mutations in IKKβ function through global changes induced by K63-linked ubiquitination and result in autocrine stimulation.PLoS One. 2018 Oct 18;13(10):e0206014. doi: 10.1371/journal.pone.0206014. eCollection 2018. PLoS One. 2018. PMID: 30335863 Free PMC article.
-
[Protein ubiquitination on the regulation of inflammatory bowel disease].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2018 Jan 25;47(1):82-88. doi: 10.3785/j.issn.1008-9292.2018.02.12. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 30146816 Free PMC article. Review. Chinese.
-
Deubiquitylating enzymes: potential target in autoimmune diseases.Inflammopharmacology. 2021 Dec;29(6):1683-1699. doi: 10.1007/s10787-021-00890-z. Epub 2021 Nov 18. Inflammopharmacology. 2021. PMID: 34792672 Review.
References
-
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. - PubMed
-
- Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-kappaB regulatory pathways. Annu Rev Biochem. 2009;78:769–796. - PubMed
-
- Hicke L, Schubert HL, Hill CP. Ubiquitin-binding domains. Nat Rev Mol Cell Biol. 2005;6:610–621. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

