Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV
- PMID: 23308151
- PMCID: PMC3538641
- DOI: 10.1371/journal.pone.0053138
Cell-to-cell transmission can overcome multiple donor and target cell barriers imposed on cell-free HIV
Abstract
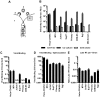
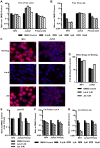
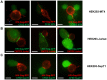
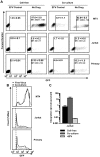
Virus transmission can occur either by a cell-free mode through the extracellular space or by cell-to-cell transmission involving direct cell-to-cell contact. The factors that determine whether a virus spreads by either pathway are poorly understood. Here, we assessed the relative contribution of cell-free and cell-to-cell transmission to the spreading of the human immunodeficiency virus (HIV). We demonstrate that HIV can spread by a cell-free pathway if all the steps of the viral replication cycle are efficiently supported in highly permissive cells. However, when the cell-free path was systematically hindered at various steps, HIV transmission became contact-dependent. Cell-to-cell transmission overcame barriers introduced in the donor cell at the level of gene expression and surface retention by the restriction factor tetherin. Moreover, neutralizing antibodies that efficiently inhibit cell-free HIV were less effective against cell-to-cell transmitted virus. HIV cell-to-cell transmission also efficiently infected target T cells that were relatively poorly susceptible to cell-free HIV. Importantly, we demonstrate that the donor and target cell types influence critically the extent by which cell-to-cell transmission can overcome each barrier. Mechanistically, cell-to-cell transmission promoted HIV spread to more cells and infected target cells with a higher proviral content than observed for cell-free virus. Our data demonstrate that the frequently observed contact-dependent spread of HIV is the result of specific features in donor and target cell types, thus offering an explanation for conflicting reports on the extent of cell-to-cell transmission of HIV.
Conflict of interest statement
Figures

Similar articles
-
Reduced Potency and Incomplete Neutralization of Broadly Neutralizing Antibodies against Cell-to-Cell Transmission of HIV-1 with Transmitted Founder Envs.J Virol. 2017 Apr 13;91(9):e02425-16. doi: 10.1128/JVI.02425-16. Print 2017 May 1. J Virol. 2017. PMID: 28148796 Free PMC article.
-
Capacity of Broadly Neutralizing Antibodies to Inhibit HIV-1 Cell-Cell Transmission Is Strain- and Epitope-Dependent.PLoS Pathog. 2015 Jul 9;11(7):e1004966. doi: 10.1371/journal.ppat.1004966. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26158270 Free PMC article.
-
Distinct Requirements for HIV-1 Accessory Proteins during Cell Coculture and Cell-Free Infection.Viruses. 2019 Apr 26;11(5):390. doi: 10.3390/v11050390. Viruses. 2019. PMID: 31027334 Free PMC article.
-
HIV-1 cell-to-cell transmission and broadly neutralizing antibodies.Retrovirology. 2018 Jul 28;15(1):51. doi: 10.1186/s12977-018-0434-1. Retrovirology. 2018. PMID: 30055632 Free PMC article. Review.
-
Cell-to-cell transmission of viruses.Curr Opin Virol. 2013 Feb;3(1):44-50. doi: 10.1016/j.coviro.2012.11.004. Epub 2012 Dec 5. Curr Opin Virol. 2013. PMID: 23219376 Free PMC article. Review.
Cited by
-
Stimuli-sensitive thiolated hyaluronic acid based nanofibers: synthesis, preclinical safety and in vitro anti-HIV activity.Nanomedicine (Lond). 2016 Nov;11(22):2935-2958. doi: 10.2217/nnm-2016-0103. Epub 2016 Oct 27. Nanomedicine (Lond). 2016. PMID: 27785967 Free PMC article.
-
Rab11-FIP1C Is Dispensable for HIV-1 Replication in Primary CD4+ T Cells, but Its Role Is Cell Type Dependent in Immortalized Human T-Cell Lines.J Virol. 2022 Dec 14;96(23):e0087622. doi: 10.1128/jvi.00876-22. Epub 2022 Nov 10. J Virol. 2022. PMID: 36354340 Free PMC article.
-
Bioorthogonal click labeling of an amber-free HIV-1 provirus for in-virus single molecule imaging.Cell Chem Biol. 2024 Mar 21;31(3):487-501.e7. doi: 10.1016/j.chembiol.2023.12.017. Epub 2024 Jan 16. Cell Chem Biol. 2024. PMID: 38232732
-
How to Control HTLV-1-Associated Diseases: Preventing de Novo Cellular Infection Using Antiviral Therapy.Front Microbiol. 2018 Mar 13;9:278. doi: 10.3389/fmicb.2018.00278. eCollection 2018. Front Microbiol. 2018. PMID: 29593659 Free PMC article.
-
HIV-1 capsid stability enables inositol phosphate-independent infection of target cells and promotes integration into genes.PLoS Pathog. 2023 Jun 2;19(6):e1011423. doi: 10.1371/journal.ppat.1011423. eCollection 2023 Jun. PLoS Pathog. 2023. PMID: 37267431 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

