Glutamate-dependent neuroglial calcium signaling differs between young and adult brain
- PMID: 23307741
- PMCID: PMC3569008
- DOI: 10.1126/science.1226740
Glutamate-dependent neuroglial calcium signaling differs between young and adult brain
Abstract
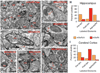
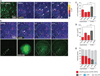
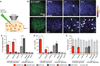
An extensive literature shows that astrocytes exhibit metabotropic glutamate receptor 5 (mGluR5)-dependent increases in cytosolic calcium ions (Ca(2+)) in response to glutamatergic transmission and, in turn, modulate neuronal activity by their Ca(2+)-dependent release of gliotransmitters. These findings, based on studies of young rodents, have led to the concept of the tripartite synapse, in which astrocytes actively participate in neurotransmission. Using genomic analysis, immunoelectron microscopy, and two-photon microscopy of astrocytic Ca(2+) signaling in vivo, we found that astrocytic expression of mGluR5 is developmentally regulated and is undetectable after postnatal week 3. In contrast, mGluR3, whose activation inhibits adenylate cyclase but not calcium signaling, was expressed in astrocytes at all developmental stages. Neuroglial signaling in the adult brain may therefore occur in a manner fundamentally distinct from that exhibited during development.
Figures
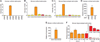
Comment in
-
Neuroscience. Developmental refining of neuroglial signaling?Science. 2013 Jan 11;339(6116):152-3. doi: 10.1126/science.1233208. Science. 2013. PMID: 23307730 No abstract available.
-
Glia: an astrocytic generation gap.Nat Rev Neurosci. 2013 Mar;14(3):157. doi: 10.1038/nrn3446. Epub 2013 Jan 30. Nat Rev Neurosci. 2013. PMID: 23361384 No abstract available.
Similar articles
-
Neuroscience. Developmental refining of neuroglial signaling?Science. 2013 Jan 11;339(6116):152-3. doi: 10.1126/science.1233208. Science. 2013. PMID: 23307730 No abstract available.
-
Receptor-selective diffusion barrier enhances sensitivity of astrocytic processes to metabotropic glutamate receptor stimulation.Sci Signal. 2012 Apr 3;5(218):ra27. doi: 10.1126/scisignal.2002498. Sci Signal. 2012. PMID: 22472649
-
Glutamate activates c-fos in glial cells via a novel mechanism involving the glutamate receptor subtype mGlu5 and the transcriptional repressor DREAM.Glia. 2007 Feb;55(3):328-40. doi: 10.1002/glia.20464. Glia. 2007. PMID: 17120244
-
Astrocytes control neuronal excitability in the nucleus accumbens.ScientificWorldJournal. 2007 Nov 2;7:89-97. doi: 10.1100/tsw.2007.195. ScientificWorldJournal. 2007. PMID: 17982581 Free PMC article. Review.
-
Astrocytic mGluR5 and the tripartite synapse.Neuroscience. 2016 May 26;323:29-34. doi: 10.1016/j.neuroscience.2015.03.063. Epub 2015 Apr 3. Neuroscience. 2016. PMID: 25847307 Review.
Cited by
-
Activating mGlu3 Metabotropic Glutamate Receptors Rescues Schizophrenia-like Cognitive Deficits Through Metaplastic Adaptations Within the Hippocampus.Biol Psychiatry. 2021 Sep 15;90(6):385-398. doi: 10.1016/j.biopsych.2021.02.970. Epub 2021 Mar 6. Biol Psychiatry. 2021. PMID: 33965197 Free PMC article.
-
Aldh1L1 is expressed by postnatal neural stem cells in vivo.Glia. 2013 Sep;61(9):1533-41. doi: 10.1002/glia.22539. Epub 2013 Jul 8. Glia. 2013. PMID: 23836537 Free PMC article.
-
Alterations in Astrocytic Regulation of Excitation and Inhibition by Stress Exposure and in Severe Psychopathology.J Neurosci. 2022 Sep 7;42(36):6823-6834. doi: 10.1523/JNEUROSCI.2410-21.2022. J Neurosci. 2022. PMID: 38377014 Free PMC article. Review.
-
Of changing glial responses, modular interactions, and why a worm may turn.J Gen Physiol. 2013 Mar;141(3):273-4. doi: 10.1085/jgp.201310970. J Gen Physiol. 2013. PMID: 23440274 Free PMC article. No abstract available.
-
Cross-Talk of the CNS With Immune Cells and Functions in Health and Disease.Front Neurol. 2021 May 31;12:672455. doi: 10.3389/fneur.2021.672455. eCollection 2021. Front Neurol. 2021. PMID: 34135852 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

