Molecular disruption of the power stroke in the ATP-binding cassette transport protein MsbA
- PMID: 23306205
- PMCID: PMC3591591
- DOI: 10.1074/jbc.M112.430074
Molecular disruption of the power stroke in the ATP-binding cassette transport protein MsbA
Abstract
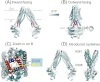
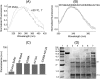
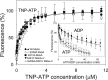
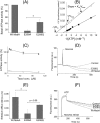
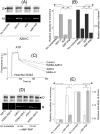
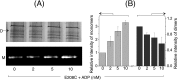
ATP-binding cassette transporters affect drug pharmacokinetics and are associated with inherited human diseases and impaired chemotherapeutic treatment of cancers and microbial infections. Current alternating access models for ATP-binding cassette exporter activity suggest that ATP binding at the two cytosolic nucleotide-binding domains provides a power stroke for the conformational switch of the two membrane domains from the inward-facing conformation to the outward-facing conformation. In outward-facing crystal structures of the bacterial homodimeric ATP-binding cassette transporters MsbA from gram-negative bacteria and Sav1866 from Staphylococcus aureus, two transmembrane helices (3 and 4) in the membrane domains have their cytoplasmic extensions in close proximity, forming a tetrahelix bundle interface. In biochemical experiments on MsbA from Escherichia coli, we show for the first time that a robust network of inter-monomer interactions in the tetrahelix bundle is crucial for the transmission of nucleotide-dependent conformational changes to the extracellular side of the membrane domains. Our observations are the first to suggest that modulation of tetrahelix bundle interactions in ATP-binding cassette exporters might offer a potent strategy to alter their transport activity.
Figures



Similar articles
-
Dissection of the conformational cycle of the multidrug/lipidA ABC exporter MsbA.Proteins. 2010 Nov 1;78(14):2867-72. doi: 10.1002/prot.22813. Proteins. 2010. PMID: 20715055
-
Substrate binding stabilizes a pre-translocation intermediate in the ATP-binding cassette transport protein MsbA.J Biol Chem. 2013 Jul 26;288(30):21638-47. doi: 10.1074/jbc.M113.485714. Epub 2013 Jun 13. J Biol Chem. 2013. PMID: 23766512 Free PMC article.
-
The conformational transition pathway of ATP binding cassette transporter MsbA revealed by atomistic simulations.J Biol Chem. 2010 Jan 29;285(5):3053-63. doi: 10.1074/jbc.M109.056432. Epub 2009 Dec 7. J Biol Chem. 2010. PMID: 19996093 Free PMC article.
-
Structure and mechanism of ABC transporter proteins.Curr Opin Struct Biol. 2007 Aug;17(4):412-8. doi: 10.1016/j.sbi.2007.07.003. Epub 2007 Aug 27. Curr Opin Struct Biol. 2007. PMID: 17723295 Review.
-
ECF-Type ATP-Binding Cassette Transporters.Annu Rev Biochem. 2019 Jun 20;88:551-576. doi: 10.1146/annurev-biochem-013118-111705. Epub 2019 Nov 28. Annu Rev Biochem. 2019. PMID: 30485755 Review.
Cited by
-
Probing the allosteric NBD-TMD crosstalk in the ABC transporter MsbA by solid-state NMR.Commun Biol. 2024 Jan 5;7(1):43. doi: 10.1038/s42003-023-05617-0. Commun Biol. 2024. PMID: 38182790 Free PMC article.
-
A Conserved Motif in Intracellular Loop 1 Stabilizes the Outward-Facing Conformation of TmrAB.J Mol Biol. 2021 Aug 6;433(16):166834. doi: 10.1016/j.jmb.2021.166834. Epub 2021 Jan 29. J Mol Biol. 2021. PMID: 33524413 Free PMC article.
-
Breaking down the cell wall: Still an attractive antibacterial strategy.Front Microbiol. 2022 Sep 23;13:952633. doi: 10.3389/fmicb.2022.952633. eCollection 2022. Front Microbiol. 2022. PMID: 36212892 Free PMC article. Review.
-
Native mass spectrometry and structural studies reveal modulation of MsbA-nucleotide interactions by lipids.Nat Commun. 2024 Jul 15;15(1):5946. doi: 10.1038/s41467-024-50350-9. Nat Commun. 2024. PMID: 39009687 Free PMC article.
-
Optimization of Membrane Protein TmrA Purification Procedure Guided by Analytical Ultracentrifugation.Membranes (Basel). 2021 Oct 12;11(10):780. doi: 10.3390/membranes11100780. Membranes (Basel). 2021. PMID: 34677546 Free PMC article.
References
-
- Gottesman M. M., Fojo T., Bates S. E. (2002) Multidrug resistance in cancer. Role of ATP-dependent transporters. Nat. Rev. Cancer 2, 48–58 - PubMed
-
- Szakács G., Paterson J. K., Ludwig J. A., Booth-Genthe C., Gottesman M. M. (2006) Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234 - PubMed
-
- Higgins C. F. (1992) ABC transporters. From microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 - PubMed
-
- Borst P., Elferink R. O. (2002) Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71, 537–592 - PubMed
-
- Marquez B., Van Bambeke F. (2011) ABC multidrug transporters. Target for modulation of drug pharmacokinetics and drug-drug interactions. Curr. Drug Targets 12, 600–620 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

