RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid
- PMID: 23306201
- PMCID: PMC3576080
- DOI: 10.1074/jbc.M112.413492
RegB kinase activity is repressed by oxidative formation of cysteine sulfenic acid
Abstract
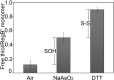
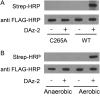
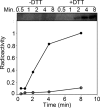
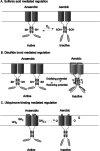
RegB/RegA comprise a global redox-sensing signal transduction system utilized by a wide range of proteobacteria to sense environmental changes in oxygen tension. The conserved cysteine 265 in the sensor kinase RegB was previously reported to form an intermolecular disulfide bond under oxidizing conditions that converts RegB from an active dimer into an inactive tetramer. In this study, we demonstrate that a stable sulfenic acid (-SOH) derivative also forms at Cys-265 in vitro and in vivo when RegB is exposed to oxygen. This sulfenic acid modification is reversible and stable in the air. Autophosphorylation assay shows that reduction of the SOH at Cys-265 to a free thiol (SH) can increase RegB kinase activity in vitro. Our results suggest that a sulfenic acid modification at Cys-265 performs a regulatory role in vivo and that it may be the major oxidation state of Cys-265 under aerobic conditions. Cys-265 thus functions as a complex redox switch that can form multiple thiol modifications in response to different redox signals to control the kinase activity of RegB.
Figures



Similar articles
-
Signal transduction by the global regulator RegB is mediated by a redox-active cysteine.EMBO J. 2003 Sep 15;22(18):4699-708. doi: 10.1093/emboj/cdg461. EMBO J. 2003. PMID: 12970182 Free PMC article.
-
RegB/RegA, a highly conserved redox-responding global two-component regulatory system.Microbiol Mol Biol Rev. 2004 Jun;68(2):263-79. doi: 10.1128/MMBR.68.2.263-279.2004. Microbiol Mol Biol Rev. 2004. PMID: 15187184 Free PMC article. Review.
-
Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB.J Biol Chem. 2006 Mar 10;281(10):6768-75. doi: 10.1074/jbc.M509687200. Epub 2006 Jan 5. J Biol Chem. 2006. PMID: 16407278 Free PMC article.
-
RegB/RegA, a global redox-responding two-component system.Adv Exp Med Biol. 2008;631:131-48. doi: 10.1007/978-0-387-78885-2_9. Adv Exp Med Biol. 2008. PMID: 18792686 Review.
-
Structural and functional analyses of photosynthetic regulatory genes regA and regB from Rhodovulum sulfidophilum, Roseobacter denitrificans, and Rhodobacter capsulatus.J Bacteriol. 1999 Jul;181(14):4205-15. doi: 10.1128/JB.181.14.4205-4215.1999. J Bacteriol. 1999. PMID: 10400577 Free PMC article.
Cited by
-
Endogenous SO2-dependent Smad3 redox modification controls vascular remodeling.Redox Biol. 2021 May;41:101898. doi: 10.1016/j.redox.2021.101898. Epub 2021 Feb 18. Redox Biol. 2021. PMID: 33647858 Free PMC article.
-
XRE transcription factors conserved in Caulobacter and φCbK modulate adhesin development and phage production.PLoS Genet. 2023 Nov 16;19(11):e1011048. doi: 10.1371/journal.pgen.1011048. eCollection 2023 Nov. PLoS Genet. 2023. PMID: 37972151 Free PMC article.
-
Characterization of a Glycyl Radical Enzyme Bacterial Microcompartment Pathway in Rhodobacter capsulatus.J Bacteriol. 2019 Feb 11;201(5):e00343-18. doi: 10.1128/JB.00343-18. Print 2019 Mar 1. J Bacteriol. 2019. PMID: 30510145 Free PMC article.
-
Triphenylphosphonium-Derived Protein Sulfenic Acid Trapping Agents: Synthesis, Reactivity, and Effect on Mitochondrial Function.Chem Res Toxicol. 2019 Mar 18;32(3):526-534. doi: 10.1021/acs.chemrestox.8b00385. Epub 2019 Mar 4. Chem Res Toxicol. 2019. PMID: 30784263 Free PMC article.
-
Redox Brake Regulator RedB and FnrL Function as Yin-Yang Regulators of Anaerobic-Aerobic Metabolism in Rhodobacter capsulatus.Microbiol Spectr. 2022 Oct 26;10(5):e0235422. doi: 10.1128/spectrum.02354-22. Epub 2022 Sep 15. Microbiol Spectr. 2022. PMID: 36106752 Free PMC article.
References
-
- Sganga M. W., Bauer C. E. (1992) Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 68, 945–954 - PubMed
-
- Inoue K., Kouadio J. L., Mosley C. S., Bauer C. E. (1995) Isolation and in vitro phosphorylation of sensory transduction components controlling anaerobic induction of light harvesting and reaction center gene expression in Rhodobacter capsulatus. Biochemistry 34, 391–396 - PubMed
-
- Bird T. H., Du S., Bauer C. E. (1999) Autophosphorylation, phosphotransfer, and DNA-binding properties of the RegB/RegA two-component regulatory system in Rhodobacter capsulatus. J. Biol. Chem. 274, 16343–16348 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

