Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells
- PMID: 23305731
- PMCID: PMC3591793
- DOI: 10.1182/blood-2012-07-445189
Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells
Abstract
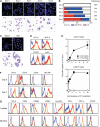
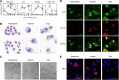
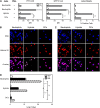
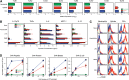
Neutrophils have been reported to acquire surface expression of MHC class II and co-stimulatory molecules as well as T-cell stimulatory activities when cultured with selected cytokines. However, cellular identity of those unusual neutrophils showing antigen presenting cell (APC)-like features still remains elusive. Here we show that both immature and mature neutrophils purified from mouse bone marrow differentiate into a previously unrecognized "hybrid" population showing dual properties of both neutrophils and dendritic cells (DCs) when cultured with granulocyte macrophage-colony-stimulating factor but not with other tested growth factors. The resulting hybrid cells express markers of both neutrophils (Ly6G, CXCR2, and 7/4) and DCs (CD11c, MHC II, CD80, and CD86). They also exhibit several properties typically reserved for DCs, including dendritic morphology, probing motion, podosome formation, production of interleukin-12 and other cytokines, and presentation of various forms of foreign protein antigens to naïve CD4 T cells. Importantly, they retain intrinsic abilities of neutrophils to capture exogenous material, extrude neutrophil extracellular traps, and kill bacteria via cathelicidin production. Not only do our results reinforce the notion that neutrophils can acquire APC-like properties, they also unveil a unique differentiation pathway of neutrophils into neutrophil-DC hybrids that can participate in both innate and adaptive immune responses.
Figures

Similar articles
-
Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice.Blood. 2013 Mar 7;121(10):1690-700. doi: 10.1182/blood-2012-07-445197. Epub 2013 Jan 10. Blood. 2013. PMID: 23305733 Free PMC article.
-
Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics.J Exp Med. 1998 Apr 6;187(7):1019-28. doi: 10.1084/jem.187.7.1019. J Exp Med. 1998. PMID: 9529318 Free PMC article.
-
Neutrophil plasticity: acquisition of phenotype and functionality of antigen-presenting cell.J Leukoc Biol. 2015 Oct;98(4):489-96. doi: 10.1189/jlb.1MR1014-502R. Epub 2015 Jan 28. J Leukoc Biol. 2015. PMID: 25632045 Review.
-
Reciprocal regulation of development of neutrophil-dendritic cell hybrids in mice by IL-4 and interferon-gamma.PLoS One. 2013 Nov 21;8(11):e82929. doi: 10.1371/journal.pone.0082929. eCollection 2013. PLoS One. 2013. PMID: 24278484 Free PMC article.
-
Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response.FEMS Immunol Med Microbiol. 2000 Apr;27(4):313-20. doi: 10.1111/j.1574-695X.2000.tb01445.x. FEMS Immunol Med Microbiol. 2000. PMID: 10727887 Review.
Cited by
-
Neutrophil subtypes shape HIV-specific CD8 T-cell responses after vaccinia virus infection.NPJ Vaccines. 2021 Apr 12;6(1):52. doi: 10.1038/s41541-021-00314-7. NPJ Vaccines. 2021. PMID: 33846352 Free PMC article.
-
Mechanisms of interferon-γ production by neutrophils and its function during Streptococcus pneumoniae pneumonia.Am J Respir Cell Mol Biol. 2015 Mar;52(3):349-64. doi: 10.1165/rcmb.2013-0316OC. Am J Respir Cell Mol Biol. 2015. PMID: 25100610 Free PMC article.
-
A late-lineage murine neutrophil precursor population exhibits dynamic changes during demand-adapted granulopoiesis.Sci Rep. 2017 Jan 6;7:39804. doi: 10.1038/srep39804. Sci Rep. 2017. PMID: 28059162 Free PMC article.
-
Novel peptide-based oncolytic vaccine for enhancement of adaptive antitumor immune response via co-engagement of innate Fcγ and Fcα receptors.J Immunother Cancer. 2024 Mar 8;12(3):e008342. doi: 10.1136/jitc-2023-008342. J Immunother Cancer. 2024. PMID: 38458776 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
References
-
- Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33(5):657–670. - PubMed
-
- Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. - PubMed
-
- Pelletier M, Maggi L, Micheletti A, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115(2):335–343. - PubMed
-
- Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatability complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89(11):4128–4135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

