Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals
- PMID: 23300469
- PMCID: PMC3531470
- DOI: 10.1371/journal.pgen.1003170
Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals
Abstract
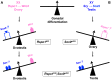
In mammals, male sex determination is governed by SRY-dependent activation of Sox9, whereas female development involves R-spondin1 (RSPO1), an activator of the WNT/beta-catenin signaling pathway. Genetic analyses in mice have demonstrated Sry and Sox9 to be both required and sufficient to induce testicular development. These genes are therefore considered as master regulators of the male pathway. Indeed, female-to-male sex reversal in XX Rspo1 mutant mice correlates with Sox9 expression, suggesting that this transcription factor induces testicular differentiation in pathological conditions. Unexpectedly, here we show that testicular differentiation can occur in XX mutants lacking both Rspo1 and Sox9 (referred to as XX Rspo1(KO)Sox9(cKO) ()), indicating that Sry and Sox9 are dispensable to induce female-to-male sex reversal. Molecular analyses show expression of both Sox8 and Sox10, suggesting that activation of Sox genes other than Sox9 can induce male differentiation in Rspo1(KO)Sox9(cKO) mice. Moreover, since testis development occurs in XY Rspo1(KO)Sox9(cKO) mice, our data show that Rspo1 is the main effector for male-to-female sex reversal in XY Sox9(cKO) mice. Thus, Rspo1 is an essential activator of ovarian development not only in normal situations, but also in sex reversal situations. Taken together these data demonstrate that both male and female sex differentiation is induced by distinct, active, genetic pathways. The dogma that considers female differentiation as a default pathway therefore needs to be definitively revised.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
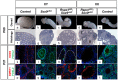
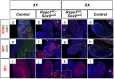
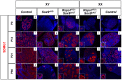
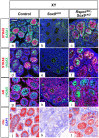

Similar articles
-
Gadd45g is required for timely Sry expression independently of RSPO1 activity.Reproduction. 2022 Apr 22;163(6):333-340. doi: 10.1530/REP-21-0443. Reproduction. 2022. PMID: 35315790 Free PMC article.
-
Sox8 and Sox9 act redundantly for ovarian-to-testicular fate reprogramming in the absence of R-spondin1 in mouse sex reversals.Elife. 2020 May 26;9:e53972. doi: 10.7554/eLife.53972. Elife. 2020. PMID: 32450947 Free PMC article.
-
Inefficient Sox9 upregulation and absence of Rspo1 repression lead to sex reversal in the B6.XYTIR mouse gonad†.Biol Reprod. 2024 May 9;110(5):985-999. doi: 10.1093/biolre/ioae018. Biol Reprod. 2024. PMID: 38376238
-
R-spondin1, WNT4, and the CTNNB1 signaling pathway: strict control over ovarian differentiation.Reproduction. 2014 Dec;148(6):R97-110. doi: 10.1530/REP-14-0177. Epub 2014 Sep 3. Reproduction. 2014. PMID: 25187620 Review.
-
Genetics of ovarian differentiation: Rspo1, a major player.Sex Dev. 2008;2(4-5):219-27. doi: 10.1159/000152038. Epub 2008 Nov 5. Sex Dev. 2008. PMID: 18987496 Review.
Cited by
-
Current insight into the transient X-zone in the adrenal gland cortex.Vitam Horm. 2024;124:297-339. doi: 10.1016/bs.vh.2023.05.003. Epub 2023 Jul 12. Vitam Horm. 2024. PMID: 38408801 Free PMC article. Review.
-
Becoming female: Ovarian differentiation from an evolutionary perspective.Front Cell Dev Biol. 2022 Sep 7;10:944776. doi: 10.3389/fcell.2022.944776. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158204 Free PMC article. Review.
-
Pervasive male-biased expression throughout the germline-specific regions of the sea lamprey genome supports key roles in sex differentiation and spermatogenesis.Commun Biol. 2022 May 10;5(1):434. doi: 10.1038/s42003-022-03375-z. Commun Biol. 2022. PMID: 35538209 Free PMC article.
-
Gadd45g is required for timely Sry expression independently of RSPO1 activity.Reproduction. 2022 Apr 22;163(6):333-340. doi: 10.1530/REP-21-0443. Reproduction. 2022. PMID: 35315790 Free PMC article.
-
Induction of meiosis by embryonic gonadal somatic cells differentiated from pluripotent stem cells.Stem Cell Res Ther. 2021 Dec 20;12(1):607. doi: 10.1186/s13287-021-02672-4. Stem Cell Res Ther. 2021. PMID: 34930450 Free PMC article.
References
-
- Gubbay J, Collignon J, Koopman P, Capel B, Economou A, et al. (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346: 245–250. - PubMed
-
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, et al. (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346: 240–244. - PubMed
-
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121. - PubMed
-
- Sekido R, Lovell-Badge R (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453: 930–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

